Abstract
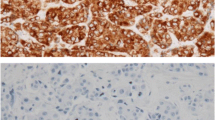
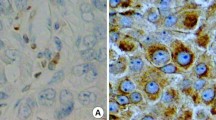
BCL-2 protein plays a pivotal role in overriding programmed cell death (apoptosis), thus favouring a prolonged survival of normal and neoplastic cells. Expression of the bcl-2 gene has been documented in some human tumours (non-Hodgkin's lymphomas and prostatic adenocarcinomas), but findings in breast carcinomas have not been reported. We have used the monoclonal antibody 124 to investigate BCL-2 expression in 212 breast carcinomas, and to correlate it with the oestrogen (ER), progesterone (PR) and epidermal growth factor receptor (EGFR) status, and with other clinicopathological variables including tumour type, grade, stage, growth fraction (as evaluated by Ki-67 immunostaining), and p53 accumulation. Of the 212 carcinomas, 173 (81.6%) exhibited BCL-2 immunoreactivity in more than 25% of the neoplastic cells. BCL-2 immunoreactivity was strongly correlated with ER and PR expression (P<0.00001), with the lobular type (P=0.012) and with better differentiated neoplasms (P=0.00003), whereas it was inversely correlated with EGFR (P<0.00001), p53 (P=0.0004) and Ki-67 (P=0.0002) immunoreactivities. No association was found with tumour stage (T and N categories). We conclude that bcl-2 expression in breast cancers is related to the oestrogen-dependent transcription pathway.
Similar content being viewed by others
References
Bartek J, Bartkova J, Vojtesek B, Staskova Z, Lukas J, Reijthar A, Kovarik J, Midgley CA, Ganon JV, Lane DP (1991) Aberrant expression of the p53 oncoprotein is a common feature of a wide spectrum of human malignancies. Oncogene 6:1699–1703
Bhagat SKM, Mediros LJ, Weiss LM, Wang J, Raffeld M, Stetler-Stevenson M (1993) bcl-2 expression in Hodgkin's disease: correlation with the t(14;18) translocation and Epstein-Barr virus. Am J Clin Pathol 99:604–608
Bosari S, Lee AKC, Viale G, Heatley GJ, Coggi G (1992) Abnormal p53 immunoreactivity and prognosis in node negative breast carcinomas with long-term follow-up. Virchows Arch [A] 421:291–295
Butta A, Mariennan K, Flanders KC, Sacks NPM, Smith I, McKinna A, Dowsett M, Wakefield LM, Sporn MB, Baum M, Colletta AA (1992) Induction of transforming growth factor β1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res 52:4261–4264
Cattoretti G, Rilke F, Andreola S, D'Amato L, Delia D (1988) p53 expression in breast cancer. Int J Cancer 41:178–183
Cleary ML, Sklar J (1985) Nucleotide sequence of a t(14,18) chromosomal breakpoint in follicular lymphomas and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci USA 82:7439–7443
Cleary ML, Smith SD, Sklar J (1986) Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2 immunoglobulin transcript resulting from the t(14;18) translocation. Cell 47:19–28
Colombel M, Symmans F, Gil S, O'Toole M, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R (1993) Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone-refractory human prostate cancers. Am J Pathol 143:390–400
Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW (1982) Infiltrating lobular carcinoma of the breast. Histopathology 6:149–161
Doglioni C, Dell'Orto P, Coggi G, Iuzzolino P, Bontempini L, Viale G (1987) Choroid plexus tumors. An immunocytochemical study with particular reference to the coexpression of intermediate filament proteins. Am J Pathol 127:519–529
Doglioni C, Gambacorta M, Zamboni G, Coggi G, Viale G (1990) Immunocytochemical localization of progesterone receptors in endocrine cells of the human pancreas. Am J Pathol 137:999–1005
Doussis IA, Pezzella F, Lane DP, Gatter KC, Mason DY (1993) An immunocytochemical study of p53 and bcl-2 protein expression in Hodgkin's disease. Am J Clin Pathol 99:663–667
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Finlay CA, Hinds PW, Tan TH, Eliyadu D, Oren M, Levine AJ (1988) Activating mutations for transformation by p53 produce a gene product that forms an hsc 70-p53 complex with an altered half-life. Mol Cell Biol 8:531–539
Fisher B (1992) Experimental and clinical justification for the use of tamoxifen in a breast cancer prevention trial: a description of the NSABP effort. Proc Am Assoc Cancer Res 33:557–560
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Hickman JA (1992) Apoptosis induced by anticancer drugs. Cancer Metastasis Rev 11:121–139
Hiort IO, Kwan PWL, DeLellis RA (1988) Immunohistochemistry of estrogen receptor protein in paraffin sections. Am J Clin Pathol 90:559–563
Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeier SJ (1990) Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348:334–336
Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeier SJ (1991) BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA 88:6961–6965
Jordan VC, Jeng MH, Jiang SY, Yingling J, Stella AL (1992) Hormonal strategies for breast cancer: a new focus on the estrogen receptor as a therapeutic target. Semin Oncol 19:299–307
Knabbe C, Lippman ME, Wakefield L, Flanders KC, Derynk R, Dickson RB (1987) Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell 48:417–428
Korsmeyer SJ (1992) Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood 80:879–886
Kyprianou N, English HF, Davidson NE, Isaacs JT (1991) Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res 51:162–166
Lane DP (1992) p53, guardian of the genome. Nature 358:15–16
LeBrun DP, Warnke RA, Cleary ML (1993) Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol 142:743–753
Lu Q-L, Poulsom R, Wong L, Hanby AM (1993) BCL-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol 169:431–437
McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LWK, Hsieh JT, Tu SM, Campbell ML (1992) Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 52:6940–6944
Nunez G, London L, Hockenbery D, Alexander M, McKearn J, Korsmeier SJ (1990) Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol 144:3602–3610
Pezzella F, Ralfkiaer E, Gatter KC, Mason DY (1990a) The 14;18 translocation in European cases of follicular lymphoma: comparison of Southern blotting and the polymerase chain reaction. Br J Haematol 76:58–64
Pezzella F, Tse AGD, Cordell JL, Pulford KAF, Gatter KC, Mason DY (1990b) Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol 137:225–232
Pezzella F, Morrison H, Jones M, Gatter KC, Lane D, Harris AL, Mason DY (1993a) Immunohistochemical detection of p53 and bcl-2 proteins in non-Hodgkin's lymphoma. Histopathology 22:39–44
Pezzella F, Turley H, Kuzu I, Tungekar MF, Dunnill MS, Pierce CB, Harris A, Gatter KC, Mason DY (1993b) bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med 329:690–694
Rogel A, Popliker M, Webb CG, Oren M (1985) p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos and tumours. Mol Cell Biol 5:2851–2856
Vaux DL, Cory S, Adams JM (1988) Bcl-2 gene promotes haematopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335:440–442
Wilson AC, Meenokshi S, Thompson HJ (1992) Morphological responses of MOD cells to tamoxifen suggests induction of apoptosis (abstract). Proc Am Assoc Cancer Res 33:151
Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M (1991) Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352:345–347
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doglioni, C., Dei Tos, A., Laurino, L. et al. The prevalence of BCL-2 immunoreactivity in breast carcinomas and its clinicopathological correlates, with particular reference to oestrogen receptor status. Vichows Archiv A Pathol Anat 424, 47–51 (1994). https://doi.org/10.1007/BF00197392
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00197392