Summary
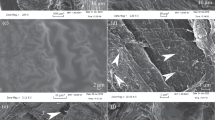
We describe by routine histology and by immunohistochemistry three phenotypically and developmentally distinct fibrocartilages associated with the Achilles tendon of the rat. All the fibrocartilages develop after birth and show significant age-related changes in the composition of their extracellular matrix. Attachment-zone fibrocartilage occurs at the insertion of the tendon on the calcaneus. It derives from the cartilage rudiment of the calcaneus and from the region where the tendon merges with the perichondrium. The extracellular matrix contain type II collagen and chondroitin sulphate. Compressive tendon fibrocartilage occurs in the deep part of the tendon where it presses against the calcaneus, and is derived by metaplasia of tendon cells. The cells label strongly for the intermediate filament vimentin, and the extracellular matrix contains chondroitin and keratan sulphates, but type II collagen only in very old animals (>2 years). Calcaneal fibrocartilage covered the posterior surface of the calcaneus where it was in contact with the Achilles tendon. It labelled intensely for type II collagen and contained chondroitin and keratan sulphates. The cells were rich in vimentin. This fibrocartilage was derived from the calcaneal perichondrium.
Similar content being viewed by others
References
Avnur Z, Geiger B (1984) Immunocytochemical localisation of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell 38:811–822
Benjamin M, Tyers RNS, Ralphs JR (1991) Age-related changes in tendon fibrocartilage. J Anat 179:127–136
Gelberman R, Goldberg V, An K-N, Banes A (1988) Tendon. In: Woo SL-Y, Buckwalter JA (eds) Injury and repair of the musculoskeletal soft tissues. American Academy of Orthopaedic Surgeons, Illinois, pp 5–40
Gillard GC, Reilly HC, Bell-Booth PG, Flint MH (1979) The influence of mechanical forces on the glycosaminoglycan content of the rabbit flexor digitorum profundus tendon. Connect Tissue Res 7:37–46
Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H (1986) Characterisation of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum 29:400–410
Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T (1986) The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated heptaor larger oligosaccharides of the poly (N-acetyllactosamine) series. Eur J Biochem 157:385–391
Ploetz E (1937) Funktioneller Bau und funktionelle Anpassung der Gleitsehne. Z Orthop 67:212–234
Ralphs JR, Benjamin M, Thornett A (1991) Cell and matrix biology of the suprapatella in the rat: a structural and immunocytochemical study of fibrocartilage in a tendon subject to compression. Anat Rec 231:167–177
Ralphs JR, Tyers RNS, Benjamin M (1992) Development of functionally distinct fibrocartilages at two sites in the quadriceps tendon of the rat: the suprapatella and the attachment to the patella. Anat Embryol 185:181–187
Rooney P, Archer CW, Wolpert L (1984) Morphogenesis of cartilaginous long bone rudiments. In: Trelstad RL (ed) The role of extracellular matrix in development. Liss, New York, pp 305–322
Stilwell DL, Gray DJ (1954) The structure of bony surfaces in contact with tendons. Anat Rec 118:358–359
Vogel KG, Koob TJ (1989) Structural specialization in tendons under compression. Int Rev Cytol 115:267–293
Woo S, Maynard J, Butler D, Lyon R, Torzilli P, Akeson W, Cooper R, Oakes B (1988) Ligament, tendon, and joint capsule insertions to bone. In: Woo SL-Y, Buckwalter JA (eds) Injury and repair of the musculoskeletal soft tissues. American Academy of Orthopaedic Surgeons, Illinois, pp 133–166
Zanetti M, Ratcliffe A, Watt FM (1985) Two subpopulations of differentiated chondrocytes identified with a monoclonal antibody to keratan sulfate. J Cell Biol 101:53–59
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rufai, A., Benjamin, M. & Ralphs, J.R. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol 186, 611–618 (1992). https://doi.org/10.1007/BF00186984
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186984