Abstract
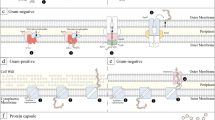
Carbohydrates are universally present on the surface of living cells. On eukaryotic cells, many different carbohydrates are attached as glycoproteins and glycolipids; the oligosaccharide moieties are known to act as receptors and it seems likely that they play an important role in cell-to-cell recognition processes. Polysaccharide capsules, in prokaryotes characteristically composed of repeating oligosaccharides, are found on the surface of many bacteria. These capsules are typically composed of only one polysaccharide and lie outside the outer membrane of gram-negative cells and the peptidoglycan layer of gram-positive cells. In general, individual bacteria do not exhibit variation of these antigens as has been described for the variant glycoproteins of trypanosomes (Cross1978). Comprising 99% water, these highly hydrated, polyanionic polysaccharide capsules serve many functions. These include determining access of molecules and ions to the bacterial cell envelope and the cytoplasmic membrane, the promotion of adherence to the surfaces of inanimate objects or living cells and the formation of biofilms and microcolonies (Costerton and Irwin1981). Among certain gram-positive and gram-negative bacteria, capsules have evolved distinctive structural and functional characteristics which are of cardinal importance in the pathogenesis of infections of animals, plants and insects (Sutherland1977).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Allan I, Loeb MR, Moxon ER (1987) Limited genetic diversity of Haemophilus influenzae (type b). Microb Pathogen 2: 139–145
Alper C, Abramson N, Johnston RB (1970) Increased susceptibility to infection associated with abnormalities of complement-mediated functions and of the third component of complement (C3). N Engl J Med 282: 349–354
Anderson PW, Johnston RB Jr, Smith DH (1972) Human serum activities against Haemophilus influenzae type b. J Clin Invest 51: 31–38
Anderson PW, Inzana T, Pichichero M (1985) Surface factors and nasopharyngeal colonization by Hemophilus influenzae B. In: Jackson GG, Thomas H (eds) The pathogenesis of bacterial infections, Springer, Berlin Heidelberg New York
Anderson RM, May RM (1982) Population biology of infectious diseases. Springer, Berlin Heidelberg New York
Branefors-Helander P, Erbing C, Kenne L, Lindberg B. (1976) Structural studies of the capsular antigen from Haemophilus influenzae type b. Acta Chem. Scand. 30: 276–277
Brown EJ, Joiner KA, Gaither TA, Hammer CH, Frank MM (1983) The interaction of C3b bound to pneumococci with factor H (beta 1H globulin), factor I (C3b/C4b inactivator) and properdin factor B of the human complement system. J Immunol 131: 409–415
Byrd RA, Egan W, Summers MF (1987) New N.M.R.-spectroscopic approaches for structural studies of polysaccharides: application to the Haemophilus influenzae type a capsular polysaccharide. Carbohydr Res 166: 47–58
Catlin BW, Bendler JW III, Goodgal SH (1972) The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol 70: 411–422
Costerton JW, Irwin RT (1981) The bacterial glycocalyx in nature and disease. Annu Rev Microbiol 35: 299–324
Costerton JW, Cheng KJ, Geesey GC, Ladd TI, Nickel JC, Dasgupta M, Morrie TJ (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41: 435–464
Crisel RM, Baker RS, Dorman DE (1975) Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J Biol Chem 250:4926–4930
Cross GAM (1978) Antigenic variation in trypanosomes. Proc Soc Lond [Biol] 20: 55–72
Edwards MS, Nicholson-Weller A, Baker CJ, Kasper DL (1980) The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med 151: 1275–1287
Egan W, Tsui F-P, Climenson PA, Schneerson R (1980 a) Structural and immunological studies of the Haemophilus influenzae type C capsular polysaccharide. Carbohydr. Res. 80: 305–316
Egan W, Tsui F-P, Schneerson R (1980 b) Structural studies of the Haemophilus influenzae type f capsular polysaccharide. Carbohydr Res 79: 271–277
Ely S, Tippett J, Moxon ER (1989) Identification and Characterization of a serotype b-specific segment of the Haemophilus influenzae genome. Infect Immun 51: (in press)
Fearon DT (1978) Regulation by membrane sialic acid of ß1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci USA 75: 1971–1975
Fielder AHL, Walport MJ, Batchelor JR, Rynes RI, Black CM, Dordi IA, Hughes GVR (1983) Family study of the major histocompatibility complex in patients with systemic lupus erythematosus: importance of null alleles of C4A and C4B in determining disease susceptibility. Br Med J 286: 425
Finne J (1982) Occurrence of unique polysialosyl carbohydrate units in glycoprotein of developing brain. J Biol Chem 257: 11966–11970
Gigliotti F, Insell RA (1983) Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis 146: 249–254
Gold R (1985) Prevention of bacterial meningitis by immunological means. In: Sande MA, Smith AL, Root RK Bacterial meningitis. Livingstone, Edinburgh, pp 105–122
Gotschlich EC, Fraser BA, Nishimura O, Robbins JB, Liu TY (1981) Lipid a capsular polysaccharide of gram-negative bacteria. J Biol Chem 256: 8915–8921
Hauptmann G, Goetz J, Uring-Lambert B, Grosshans E (1986) Complement deficiences. II. The fourth component. Prog Allergy 39: 232–249
Hoiseth SK, Connelly CJ, Moxon ER (1985) Genetics of spontaneous, high-frequency loss of b capsule expression in Haemophilus influenzae. Infect Immun 49: 389–395
Hoiseth SK, Moxon ER, Silver RP (1986) Genes involved in Haemophilus influenzae type b capsule expression are part of an 18-kilobase tandem duplication. Proc Natl Acad Sci USA 83: 1106–1110
Hoogerhout P, Evenberg D, van Boeskel CAA, Poolman JT, Beuvery EC, van der Marel GA, van Boom JH (1987) Synthesis of fragments of the capsular polysaccharide of HiTb comprising 2 or 3 repeating units. Tetrahedron Lett 28: 1953–1956
Horwitz MA, Silverstein SC (1980) Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest 65: 82–94
Kasper DL (1986) Bacterial capsule — old dogmas and new tricks. J Infect Dis 153: 407–415
Kroll JS, Moxon ER (1988) Capsulation and gene copy — number at the cap locus of Haemophilus influenzae type b. J Bacteriol 170: 859–864
Kroll JS, Hopkins I, Moxon ER (1988) Capsule loss in Haemophilus influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell 53: 347–356
Kuo JS-C, Doelling VW, Graveline JF, McCoy DW (1985) Evidence for covalent attachment of phospholipid to the capsular polysaccharide of Haemophilus influenzae type b. J Bacteriol 163: 769–773
Law SKA, Dodds AW, Porter RR (1984) A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J 3: 1819–1823
Lifely MR, Moreno C, Lindon JC (1987) An integrated molecular and immunological approach towards a meningococcal group B vaccine. Vaccine 5: 11–26
Lipuma JJ, Gilsdorf JR (1987) Role of capsule in adherence of Haemophilus influenzae type b to human buccal epithelial cells. Infect Immun 55: 2308–2310
Marcus RL, Shin HS, Mayer MM (1971) An alternate complement pathway: C3 clearing activity not due to C4 2a on endotoxic lipopolysaccharide after treatment with guinea pig serum: relation to properdin (complement components). Proc Natl Acad Sci USA 68: 1351
Moxon ER (1986) The carrier state: Haemophilus influenzae. J Antimicrob Chemother 18A 17–24
Moxon ER, Murphy PA (1978) Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc Natl Acad Sci USA 75: 1534–1536
Moxon ER, Vaughn KA (1981) The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis 143: 517–534
Moxon ER, Winkelstein JA (1988) Interaction of Haemophilus influenzae with complement. In: Cabello FC, Pruzzo C (eds) Bacteria, complement and the plagocytic cell. Springer Verlag Berlin Heidelberg New York Tokyo, pp 177–186
Musser JM, Granoff DM, Pattison PE, Selander RK (1985) A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc Natl Acad Sci USA 82: 5078–5082
Musser JM, Kroll JS, Moxon ER, Selander RK (1988) Clonal population structure of encapsulated Haemophilus influenzae. Infect Immun 56 : 1837–1845
Pangburn MK, Schreiber RD, Muller-Eberhard HJ (1977) Human complement C3b inactivator. Isolation, characterization and demonstration of an absolute requirement for serum protein ß1H for cleavage of C3b and C4b in isolation. J Exp Med 146: 257
Petersdorf RG, Luttrell CN (1962) Studies on the pathogenesis of meningitis. I. Intrathecal injection. J Clin Invest 41: 311–319
Pittman M (1931) Variation and type specificity in the bacterial species Haemophilus influenzae. J Exp Med 53:471–493
Ponder E (1928) The physical factors involved in phagocytosis. Protoplasma 3: 611
Quinn P, Crosson FJ, Winkelstein J, Moxon ER (1977) Activation of the alternative complement pathway by H. influenzae type b. Infect Immun 16: 400–402
Robbins JB (1978) Vaccines for the prevention of encapsulated bacterial diseases : current status, problems and prospects for the future. Immunochemistry 15: 839–854
Rosenow EC (1907) Human pneumococcal opsonin and the antiopsonic substance in virulent pneumococci. J Infect Dis 4: 285
Rubin LG, Moxon ER (1983) Pathogenesis of bloodstream invasion with Haemophilus influenzae type b. Infect Immun 41: 280–284
Rubin LG, Zwahlen A, Moxon ER (1985) Role of intravascular replication in the pathogenesis of experimental bacteremia due to Haemophilus influenzae type b. J Infect Dis 152: 307–314
Santosham M, Moxon ER (1977) Detection and quantitation of bacteremia in childhood. J Pediatr 91: 719–721
Selander RK, Caugant DA, Ochman H, Musser JM, Gilmour MN, Whittam TS (1986) Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol 51: 873–884
Steele NP, Munson RS Jr, Granoff DM et al. (1984) Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun 44: 452–458
Stephens DS, Hoffman LH, McGee ZA (1983) Interaction of Neisseria meningitidis with human nasopharyngeal mucosa : attachment and entry into columnar epithelial cells. J Infect Dis 148: 369–376
Sutherland IW (1977) Bacterial exopolysaccharides — their nature and production. In: Sutherland IW (ed) Surface Carbohydrates of the Procaryotic cell. Academic, London
Swift AJ, Moxon ER, Zwahlen A, Winkelstein JA (1988) Complement-mediated serum activities against isogenic capsular transformants of Haemophilus influenzae. Pediatr Res (submitted)
Tarr PI, Hosea SW, Brown EJ et al. (1982) The requirement of specific anticapsular IgG for killing of Haemophilus influenzae by an alternative pathway of complement activation. J Immunol 120: 1772–1775
Toews GB, Vial WC, Hansen EJ (1985) Role of C5 and recruited neutrophils in early clearance of nontypable Haemophilus influenzae from murine lungs. Infect Immun 50: 207–212
Tsui F-P, Schneerson R, Boykins RA, Karpas AB, Egan W (1981 a) Structural and immunological studies of the Haemophilus influenzae type d capsular polysaccharide. Carbohydr Res 97: 293 to 306
Tsui F-P, Schneerson R, Egan W (1981 b) Structural studies of the Haemophilus influenzae type e capsular polysaccharide. Carbohydr Res 88: 85–92
Turk DC (1982) Clinical importance of Haemophilus influenzae — 1981. In: Sell SH, Wright PE (eds) Haemophilus influenzae. Elsevier, New York, pp 19
Van Oss, CJ, Gillman CF (1973) Phagocytosis as a surface phenomenon : influence of C1423 on the contact angle and on the phagocytosis of sensitized encapsulated bacteria. Immunol Commun 2: 415
Weller PF, Smith AL, Anderson P, Smith DH (1977) The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis 135: 34–41
White B (1938) Antibodies to pneumococcus. In: White B, Robinson ES, Barnes LA (eds) The Biology of pneumococcus. Harvard University Press, Cambridge, pp 355–426
Winkelstein JA (1981) The role of complement in the host’s defence against Streptococcus pneumoniae. Rev Infect Dis 3: 289
Winkelstein JA, Tomasz A (1978) Activation of the alternative pathway by pneumococcal cell wall teichoic acid. J Immunol 120: 174
Zwahlen A, Rubin LG, Moxon ER (1986) Contribution of lipopolysaccharide to pathogenicity of Haemophilus influenzae: comparative virulence of genetically-related strains in rats. Microb Pathogen 1: 465–473
Zwahlen A, Kroll JS, Rubin LG, Moxon ER (1989) The molecular basis of pathogenicity in Haemophilus influenzae: Comparative virulence of genetically-related capsular transformants and correlation with changes at the capsulation locus cap. Microbiol Pathogen (in press)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1990 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Moxon, E.R., Kroll, J.S. (1990). The Role of Bacterial Polysaccharide Capsules as Virulence Factors. In: Jann, K., Jann, B. (eds) Bacterial Capsules. Current Topics in Microbiology and Immunology, vol 150. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-74694-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-74694-9_4
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-74696-3
Online ISBN: 978-3-642-74694-9
eBook Packages: Springer Book Archive