Abstract
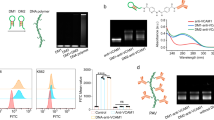
By leveraging the capacity to promote regeneration, stem cell therapies offer enormous hope for solving some of the most tragic illnesses, diseases, and tissue defects world-wide. However, a significant barrier to the effective implementation of cell therapies is the inability to target a large quantity of viable cells with high efficiency to tissues of interest. Systemic infusion is desired as it minimizes the invasiveness of cell therapy, and maximizes practical aspects of repeated doses. However, cell types such as mesenchymal stem cells exhibit a poor homing capability or lose their capacity to home following culture expansion (i.e. FASEB J 21:3197–3207, 2007; Circulation 108:863–868, 2003; Stroke: A Journal of Cerebral Circulation 32:1005–1011; Blood 104:3581–3587, 2004). To address this challenge, we have developed a simple platform technology to chemically attach cell adhesion molecules to the cell surface to improve the homing efficiency to specific tissues. This chemical approach involves a stepwise process including (1) treatment of cells with sulfonated biotinyl-N-hydroxy-succinimide to introduce biotin groups on the cell surface, (2) addition of streptavidin that binds to the biotin on the cell surface and presents unoccupied binding sites, and (3) attachment of biotinylated targeting ligands that promote adhesive interactions with vascular endothelium. Specifically, in our model system, a biotinylated cell rolling ligand, sialyl Lewisx (SLeX), found on the surface of leukocytes (i.e., the active site of the P-selectin glycoprotein ligand (PSGL-1)), is conjugated on MSC surface. The SLeX engineered MSCs exhibit a rolling response on a P-selectin coated substrate under shear stress conditions. This indicates that this approach can be used to potentially target P-selectin expressing endothelium in the more marrow or at sites of inflammation. Importantly, the surface modification has no adverse impact on MSCs’ native phenotype including their multilineage differentiation capacity, viability, proliferation, and adhesion kinetics. We anticipate that the present approach to covalently modify the cell surface and immobilize required ligands is not limited to MSCs or the SLeX ligand. Therefore, this technology should have broad implications on cell therapies that utilize systemic administration and require targeting of cells to specific tissues. The approach may also be useful to promote specific cell–cell interactions. In this protocol, we describe the conjugation of SLeX on MSC surface and methods to study cell rolling behaviors of SLeX-modified MSCs on a P-selectin coated substrate using an in vitro flow chamber assay. We also provide a brief description of cell characterization assays that can be used to examine the impact of the chemical modification regimen.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Picinich, S. C., Mishra, P. J., Mishra, P. J., Glod, J., and Banerjee, D. (2007) The therapeutic potential of mesenchymal stem cells, Cell and Tissue-Based Therapy, Expert Opinion on Biological Therapy 7, 965–973.
Burt, R. K., Loh, Y., Pearce, W., Beohar, N., Barr, W. G., Craig, R., Wen, Y., Rapp, J. A., and Kessler, J. (2008) Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases, JAMA 299, 925–936.
Abdel-Latif, A., Bolli, R., Tleyjeh, I. M., Montori, V. M., Perin, E. C., Hornung, C. A., Zuba-Surma, E. K., Al-Mallah, M., and Dawn, B. (2007) Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis, Archives of Internal Medicine 167, 989–997.
Filho Cerruti, H., Kerkis, I., Kerkis, A., Tatsui, N. H., da Costa Neves, A., Bueno, D. F., and da Silva, M. C. (2007) Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: clinical case reports, Artificial Organs 31, 268–273.
Garcia-Olmo, D., Garcia-Arranz, M., Herreros, D., Pascual, I., Peiro, C., and Rodriguez-Montes, J. A. (2005) A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation, Diseases of the Colon and Rectum 48, 1416–1423.
Maitra, B., Szekely, E., Gjini, K., Laughlin, M. J., Dennis, J., Haynesworth, S. E., and Koc, O. N. (2004) Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation, Bone Marrow Transplantation 33, 597–604.
Karp, J. M., and Leng Teo, G. S. (2009) Mesenchymal stem cell homing: the devil is in the details, Cell Stem Cell 4, 206–216.
Phinney, D. G., and Prockop, D. J. (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views, Stem Cells (Dayton, Ohio) 25, 2896–2902.
Ruster, B., Gottig, S., Ludwig, R. J., Bistrian, R., Muller, S., Seifried, E., Gille, J., and Henschler, R. (2006) Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells, Blood 108, 3938–3944.
Sackstein, R., Merzaban, J. S., Cain, D. W., Dagia, N. M., Spencer, J. A., Lin, C. P., and Wohlgemuth, R. (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone, Nature Medicine 14, 181–187.
Cheng, Z., Ou, L., Zhou, X., Li, F., Jia, X., Zhang, Y., Liu, X., Li, Y., Ward, C. A., Melo, L. G., and Kong, D. (2008) Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance, Molecular Therapy 16, 571–579.
Sarkar, D., Vemula, P. K., Teo, G. S., Spelke, D., Karnik, R., Wee le, Y., and Karp, J. M. (2008) Chemical engineering of mesenchymal stem cells to induce a cell rolling response, Bioconjugate Chemistry 19, 2105–2109.
Yago, T., Leppanen, A., Qiu, H., Marcus, W. D., Nollert, M. U., Zhu, C., Cummings, R. D., and McEver, R. P. (2002) Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow, The Journal of Cell Biology 158, 787–799.
Wiese, G., Barthel, S. R., and Dimitroff, C. J. (2009) Analysis of physiologic E-selectin-mediated leukocyte rolling on microvascular endothelium, Journal of Visualized Experiments 11, 1009–1020.
Hong, S., Lee, D., Zhang, H., Zhang, J. Q., Resvick, J. N., Khademhosseini, A., King, M. R., Langer, R., and Karp, J. M. (2007) Covalent immobilization of p-selectin enhances cell rolling, Langmuir 23, 12261–12268.
Brunk, D. K., Goetz, D. J., and Hammer, D. A. (1996) Sialyl Lewis(x)/E-selectin-mediated rolling in a cell-free system, Biophysical Journal 71, 2902–2907.
Mazo, I. B., and von Andrian, U. H. (1999) Adhesion and homing of blood-borne cells in bone marrow microvessels, Journal of Leukocyte Biology 66, 25–32.
Eniola, A. O., Willcox, P. J., and Hammer, D. A. (2003) Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs, Biophysical Journal 85, 2720–2731.
Rodgers, S. D., Camphausen, R. T., and Hammer, D. A. (2000) Sialyl Lewis(x)-mediated, PSGL-1-independent rolling adhesion on P-selectin, Biophysical Journal 79, 694–706.
Zou, X., Shinde Patil, V. R., Dagia, N. M., Smith, L. A., Wargo, M. J., Interliggi, K. A., Lloyd, C. M., Tees, D. F., Walcheck, B., Lawrence, M. B., and Goetz, D. J. (2005) PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow, American Journal of Physiology 289, C415–C424.
Zhang, M., Mal, N., Kiedrowski, M., Chacko, M., Askari, A. T., Popovic, Z. B., Koc, O. N., and Penn, M. S. (2007) SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction, FASEB J 21, 3197–3207.
Barbash, I. M., Chouraqui, P., Baron, J., Feinberg, M. S., Etzion, S., Tessone, A., Miller, L., Guetta, E., Zipori, D., Kedes, L. H., Kloner, R. A., and Leor, J. (2003) Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution, Circulation 108, 863–868.
Chen, J., Li, Y., Wang, L., Zhang, Z., Lu, D., Lu, M., and Chopp, M. (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats, Stroke: A Journal of Cerebral Circulation 32, 1005–1011.
Kawada, H., Fujita, J., Kinjo, K., Matsuzaki, Y., Tsuma, M., Miyatake, H., Muguruma, Y., Tsuboi, K., Itabashi, Y., Ikeda, Y., Ogawa, S., Okano, H., Hotta, T., Ando, K., and Fukuda, K. (2004) Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction, Blood 104, 3581–3587.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Sarkar, D., Zhao, W., Gupta, A., Loh, W.L., Karnik, R., Karp, J.M. (2011). Cell Surface Engineering of Mesenchymal Stem Cells. In: Vemuri, M., Chase, L., Rao, M. (eds) Mesenchymal Stem Cell Assays and Applications. Methods in Molecular Biology, vol 698. Humana Press. https://doi.org/10.1007/978-1-60761-999-4_35
Download citation
DOI: https://doi.org/10.1007/978-1-60761-999-4_35
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-60761-998-7
Online ISBN: 978-1-60761-999-4
eBook Packages: Springer Protocols