Abstract
Infections in every epidemic season induced by respiratory viruses, especially by the influenza virus, are the cause of many illnesses and complications which often end in death. The aim of this study was to determine the activity of influenza and influenza-like viruses in individuals aged over of 14 in Poland during the 2015/2016 epidemic season. A total of 5070 specimens taken from patients were analyzed. The presence of the influenza virus was confirmed in 40.2% of cases, among which the subtype A/H1N1/pdm09 (62.6% positive samples) predominated. The analysis of confirmed influenza and influenza-like viruses in individuals divided into four age-groups demonstrate that the highest morbidity was reported for the age ranges: 45–64 (13.1%) and 26–44 (12.6%) years. An increase in the number of influenza type B cases (23.7% positive samples), which was the main cause of morbidity in the age group 15–25 years, was noticeable. Given the epidemiological and virological data, the 2015/2016 season in Poland was characterized by increased activity of the influenza virus compared to the previous season. In the 2015/2016 season, there were more than 3.8 million cases and suspected cases of influenza and influenza-like illness, more than 15,000 hospitalizations, and up to 140 deaths.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Influenza is an infectious viral disease of the respiratory system, caused by influenza viruses belonging to Orthomyxoviridae. There are three types of the influenza virus: A, B and C, among which infections caused by type C are asymptomatic. In every epidemic season, infections are induced by influenza virus type A and B, with usually a different course of illness for either type of virus, which also depends on the patient’s age and the immune system’s efficiency (Brydak 2008). Influenza A virus is divided into subtypes, depending on the combination of the surface antigens hemagglutinin (HA) and neuraminidase (NA). There are 18 types of HA and 11 types of NA described (Wu et al. 2014). Improperly treated infections caused by influenza viruses can lead to complications and consequently to death. Therefore, it is important to confirm the presence of influenza virus in a short period of time, using molecular biology methods (Bednarska et al. 2016b), which enables to apply antiviral drugs, i.e., neuraminidase inhibitors, soon after onset of symptoms (Fiore et al. 2011; Brydak 2008).
The 2015/2016 influenza season was characterized by an increase in the number of cases and suspected cases of the influenza and influenza-like viruses, compared to the 2014/2015 season. There has also been a significant increase in deaths due to complications of influenza, the number of which was up to 135 in adult patients, compared with the mere 11 fatal cases in the 2014/2015 season.
It is meaningful that in adult patients qualified to high-risk groups, e.g., those over 65 years of age, influenza may lead to exacerbations of chronic diseases and may be characterized by an acute fulminant course (Mastalerz-Migas et al. 2015; Brydak et al. 2009). However, the percentage of vaccinated population was only 2.39% in this age range in Poland in the epidemic season in question, despite the fact that the majority of local governments make the vaccination available free of charge for people over 50 years of age (Brydak et al. 2012).
The aim of the present study was to analyze the activity influenza and influenza-like viruses in people over the age of 14 in the 2015/2016 influenza season in Poland, according to the new reporting system (Bednarska et al. 2016a).
2 Methods
2.1 Collection of Specimens
The study was approved by an institutional Ethics Committee and it was conducted in accordance with the principles for biomedical human research set by the Declaration of Helsinki.
In the 2015/2016 influenza season, lasting from week 40 (01 Oct 2015) to week 34 (28 Aug 2016), a total of 8542 clinical specimens were tested. Of those specimens, 5070 were collected from people aged over 14, taken from patients in four age-groups: 15–25, 26–44, 45–64, and over 65 years of age, collected by the Sentinel and Non-Sentinel systems. Swabs from the nose and throat, and bronchial lavage fluid were analyzed in the Department Of Influezna Research, National Influenza Center (NIC) at the National Institute of Public Health – National Institute of Hygiene (NIPH-NIH) and in 16 Voivodeship (province) Sanitary Epidemiological Stations (VSES) in Poland. Specimens typed in VSES were sent to the NIC for the confirmation of the presence of influenza virus and determination of a viral subtype using real-time PCR (qRT-PCR).
2.2 Isolation of RNA
Viral RNA was isolated from a 200 μL sample suspended in phosphate buffered saline using Maxwell 16 Total Viral Nucleic Acid Purification Kit (Promega Corporation; Madison, WI) according to the manufacturer’s instructions. The isolate was eluted in 50 μL of RNase-free water.
2.3 Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The isolated material was analyzed using the RT-PCR to confirm the presence of influenza A and B viruses. A Transcriptor One-Step RT-PCR Kit (Qiagen; Venlo, The Netherlands) was used for the RT-PCR reaction. A 20 μL reaction mix, consisting of RNase free water, buffer, primer F (5 mM), primer R (5 mM), and the transcriptor enzyme mix, was incubated with 5 μL RNA isolated from a sample. For each tested sample, an internal control amplification was performed. Positive control consisted of RNA isolated from the reference viruses A/California/7/2009(H1N1) and B/Phuket/3073/2013, recommended by the WHO for the 2015/2016 influenza season as the vaccine components. Negative control consisted of RNase-free water. Before amplification, isolated RNAs were reverse transcribed into cDNA at 50 °C for 15 min. cDNA was analyzed as follows: denaturation (1 cycle of 94 °C for 7 min), 45 cycles of amplification: denaturation at 94 °C for 10 s, annealing at 55 °C for 30 s, and elongation at 68 °C for 55 s. The reaction products were detected by gel electrophoresis.
2.4 Real-Time PCR (qRT-PCR)
qRT-PCR reactions were carried out in capillary tubes, using a Roche Light Cycler 2.0 System (Roche Diagnostics; Rotkreuz, Switzerland). The 15 μL reaction mix consisted of RNase free water, buffer, MgSO4, 0.5 mM primers F and R, 0.2 μM probes and the SuperScript III/Platinum Taq Mix (Invitrogen Life Technologies – Thermo Fisher Scientific; Carlsband, CA), incubated with 5 μL sample of RNA per capillary tube. Positive control consisted of RNA isolated from the reference A/California/7/2009(H1N1), A/Switzerland/971593/2013(H3N2), and B/Phuket/3073/2013 viruses, and RNase-free water was used for negative control. Before the amplification step, isolated RNA were reverse transcribed into cDNA at 45 °C for 15 min. The cDNA was analyzed as follows: initialization step of 1 cycle at 95 °C for 2 min, followed by 45 cycles of amplification consisting of denaturation at 95 °C for 15 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 20 s.
2.5 Conventional Multiplex RT-PCR
The presence of influenza-like viruses was confirmed in two steps by RT-PCR using RV12 ACE Detection Kit (Seegene; Seul, South Korea). The material was tested for the following respiratory viruses: influenza virus A and B, human respiratory syncytial virus A (RSV A), human respiratory syncytial virus B (RSV B), human adenovirus (AdV), human metapneumovirus (hMPV), human coronavirus 229E/NL63, human coronavirus OC43, human parainfluenza virus 1, 2, and 3 (PIV-1, PIV-2, PIV-3), and human rhinovirus A/B (RhV A/B). Random hexamer-primed cDNA synthesis products were generated using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific; Carlsband, CA), according to the manufacturer’s instructions. Each cDNA preparation was subjected to the RV12 PCR procedure according to the manufacturer’s instructions (Seegene, Seoul, South Korea). Afterward, amplicons were detected by gel electrophoresis.
3 Results
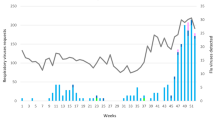
The 2015/2016 epidemic influenza season in Poland was characterized by a high number of 3,864,731 cases and suspected cases of influenza and influenza-like viruses (ILI). There were 15,312 hospitalizations and 140 deaths due to complications of influenza, including 135 deaths among people over 14 years of age. The peak incidence of ILI occurred in week 8 of 2016. During the season more than 8500 samples were tested and more than 5000 of them were collected from people aged over 14. The distribution of confirmed cases of influenza viruses by age and the viral type are shown in Figs. 1 and 2. The highest percentage of confirmations was observed in the persons aged 45–64 (13.1% of cases), less in those aged 26–44 (12.6%) and over 65 (9.9% of cases), while in the group of 15–25 years of age viruses were confirmed in 4.6% of cases. The dominant type was influenza A virus, subtype A/H1N1/pdm09. The highest number of confirmed cases of this subtype was reported in the persons aged 45–64 (n = 489), while in those aged 26–44 and over 65 the numbers of confirmed cases were comparable: 350 and 336, respectively. In the persons aged 15–25, subtype A/H1N1/pdm09 was confirmed in 66 cases. The subtype A/H1N1/ was detected in individual cases in all age-groups, with more than 10 cases present only in the persons aged over 65 (Fig. 1). Concerning the unsubtyped influenza virus type A, the number of confirmed cases was similar in all age-groups investigated, amounting to 64 cases in the persons aged 15–25, 67 cases in those aged 26–44, 70 cases in those aged 45–64, and 52 cases in those aged over 65. Concerning the subtype A/H3N2/, single cases were observed in persons aged 26–44 and 45–64 (Fig. 1).
There also were confirmed cases of influenza B in individuals over 14 years of age. This was actually the dominant type of influenza virus in persons aged 15–25. However, the highest number of cases of influenza virus type B was found in the persons aged 26–44 (n = 201), far fewer in those aged 15–25 years (n = 95), 45–64 years (n = 85), and over 65 years (n = 89) (Fig. 2).
In the 2015/2016 influenza season, there were just 16 cases of influenza-like viruses reported, with RSV (n = 6), PIV-3 (n = 4), and ADV (n = 3) reported most frequently and with individual cases of PIV 1, hCoV and RhV A/B virues.
4 Discussion
The number of 3,864,731 cases and suspected cases of influenza and influenza-like viruses in the 2015/2016 influenza epidemic season in Poland was comparable to that present in the preceding season (NIPH-NIH 2015/2016). However, the number of hospitalizations and deaths due to complications increased dramatically. The number of deaths increased from 11 in 2014/2015 to 140 in 2015/2016 (Table 1).
Despite a comparable number of cases and suspected cases of influenza and influenza-like viruses, the number of confirmed cases increased to 40.2% in 2015/2016 up from 21.2% in 2014/2015 (Bednarska et al. 2016). There also was a difference in the most frequent contagion between the two seasons, with subtype A/H1N1/pdm09 (62.6%) predominating in 2015/2016 as opposed to A/H3N2/ predominating the season before in persons over 14 years of age (Hallmann-Szelińska et al. 2016). The present study demonstrates that subtype A/H1N1/pdm09 predominated in all age groups, except for 15–24 years old persons in whom virus type B was dominant (Fig. 2). In both compared seasons, the highest incidence of influenza was reported in the age-groups of 45–64 and 26–44 years and the lowest one in the 15–24 years old persons (Fig. 1) (Bednarska et al. 2016b). Interestingly, the number of confirmed cases of influenza virus was the highest in the same age-groups of 45–64 and 26–44 years in the past 2013/2014 season (Bednarska et al. 2015). We also found that the activity of RSV and PIV-3 was increased in 2015/2016 compared with that in the season before (NIPH-NIH 2015/2016).
The following strains were included into the influenza vaccine in the 2015/2016 season: A/California/7/2009(H1N1)pdm09, A/Switzerland/9715293/2013(H3N2) and B/Phuket/3073/2013. A particular attention should be paid to the A/California/7/2009(H1N1)pdm09 strain which was a vaccine component since the 2010/2011 season, and also caused most influenza cases in persons over 14 years of age in the currently evaluated season. Given that the percentage of vaccinated population in this age range in Poland ranks at a dismally low level and has a decreasing trend from 1.95% in 2011 to 1.37% in 2015 (Epimeld 2016), it can be judged that that the recorded number of influenza cases reflects the immunodeficiency to this viral strain (Brydak 2015).
An increased number of confirmed cases of infection in the currently evaluated season was mainly caused by the influenza virus and to a lesser extent by influenza-like viruses, which may lead to a severer disease course, complications, and in consequence to death. The death as a sequelae of influenza was due usually to complications arising from the underlying chronic comorbidities, especially cardiovascular and respiratory disorders that ended up in fulminant pneumonia.
The present findings of 40.2% of confirmed cases of influenza and influenza-like viruses in the 2015/2016 influenza season show a near doubling of laboratory confirmations compared with past seasons (Bednarska et al. 2016b; Bednarska et al. 2015). That result points to substantial improvements in infection surveillance and imbued nuances in the virological and epidemiological procedures despite a drastically low and inexplicably decreasing percentage of adult, mostly middle-aged, population getting vaccinated against influenza each epidemic season in Poland. The virological data presented in this article seek to call repeat attention to the need to vaccinate against influenza as the most effective method of preventing the infection and its severe complications, death included; a need advocated by the WHO, major medical societies, and 142 National Influenza Centers worldwide.
References
Bednarska K, Hallmann-Szelińska E, Kondratiuk K, Brydak LB (2015) Evaluation of the activity of influenza and influenza-like viruses in the epidemic season. Adv Exp Med Biol 12:1–7
Bednarska K, Hallmann-Szelińska E, Kondratiuk K, Rabczenko D, Brydak LB (2016a) Novelties in influenza surveillence in Poland. Probl Hig Epidemiol 97(2):101–105
Bednarska K, Hallmann-Szelińska E, Kondratiuk K, Brydak LB (2016b) Antigenic drift of A/H3N2/virus and circulation of influenza-like viruses during the 2014/2015 influenza season in Poland. Adv Exp Med Biol 905:33–38
Brydak LB (2008) Influenza, pandemic flu, myth or real threat? Rythm, Warsaw, pp. 1–492 (in Polish)
Brydak LB (2015) Prophylaxis of influenza in general practice. Top Med Trends Guide Physician 1:9–11 (in Polish)
Brydak LB, Romanowska M, Nowak I, Ciszewski A, Bilińska ZT (2009) Antibody response to influenza vaccine in coronary artery disease: a substudy of the FLUCAD study. Med Sci Monit 15(7):PH85–PH91
Brydak LB, Roiz J, Faivre P, Reygrobellet C (2012) Implementating an influenza vaccination programme for adults aged ≥65 years in Poland. Clin Drug Investig 32:73–85
Epimeld (2016) Vaccinations in Poland in 2015. Available from: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html#05 (in Polish)
Fiore AE, Fry A, Shay D et al (2011) Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 60(RR01):1–24
Hallmann-Szelińska E, Bednarska K, Korczyńska M, Paradowska-Stankiewicz I, Brydak LB (2016) Virological characteristic of the 2014/2015 influezna season based on molecular analysis of biological material derived from I-MOVE study. Adv Exp Med Biol 857:45–40
Mastalerz-Migas A, Bujnowska-Fedak M, Brydak LB (2015) Immune efficacy of first and repeat trivalent influenza vaccine in healthy subjects and hemodialysis patients. Adv Exp Med Biol 836:47–54
NIPH-NIH (2015/2016) http://wwwold.pzh.gov.pl/oldpage/epimeld/grypa/index.htm. Accessed 22 Nov 2016
Wu Y, Wu Y, Tefsen B, Shi Y, Gao GF (2014) Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol 22(4):183–191
Acknowledgments
Funded by NIPH-NIH thematic subject 5/EM.1. The authors would like to acknowledge physicians and employees of VSESs participating in SENTINEL and non- Sentinel programs for their input into the influenza surveillance in Poland.
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kowalczyk, D., Cieślak, K., Szymański, K., Brydak, L.B. (2017). The Activity of Influenza and Influenza-like Viruses in Individuals Aged over 14 in the 2015/2016 Influenza Season in Poland. In: Pokorski, M. (eds) Respiratory System Diseases. Advances in Experimental Medicine and Biology(), vol 980. Springer, Cham. https://doi.org/10.1007/5584_2016_202
Download citation
DOI: https://doi.org/10.1007/5584_2016_202
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59497-2
Online ISBN: 978-3-319-59498-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)