Abstract
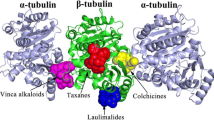
Tubulin targeting agents constitute an important class of anticancer drugs. By acting either as microtubule stabilizers or destabilizers, they disrupt microtubule dynamics, thus inducing mitotic arrest and, ultimately, cell death by apoptosis. Three different binding sites, whose exact location on tubulin has been experimentally detected, have been identified so far for antimitotic compound targeting microtubules, namely the taxoid, the colchicine and the vinka alkaloid binding site. A number of ligand- and structure-based molecular modeling studies in this field has been reported over the years, aimed at elucidating the binding modes of both stabilizing and destabilizing agent, as well as the molecular features responsible for their efficacious interaction with tubulin. Such studies are described in this review, focusing on information provided by different modeling approaches on the structural determinants of antitubulin agents and the interactions with the binding pockets on tubulin emerged as fundamental for antitumor activity.To describe molecular modeling approaches applied to date to molecules known to bind microtubules, this paper has been divided into two main parts: microtubule destabilizing (Part 1) and stabilizing (Part 2) agents. The first part includes structure-based and ligand-based approaches to study molecules targeting colchicine (1.1) and vinca alkaloid (1.2) binding sites, respectively. In the second part, the studies performed on microtubule-stabilizing antimitotic agents (MSAA) are described. Starting from the first representative compound of this class, paclitaxel, molecular modeling studies (quantitative structure-activity relationships – QSAR – and structure-based approaches), performed on natural compounds acting with the same mechanism of action and temptative common pharmacophoric hypotheses for all of these compounds, are reported.
1 Molecular Modeling on Microtubule Destabilizing Agents
1.1 Molecular Modeling on Compounds Targeting the Colchicine Binding Site
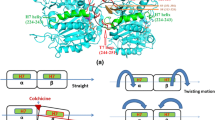
1.1.1 Colchicine Site Inhibitors
1.1.2 Ligand-Based Approaches to Colchicine Site Inhibitors
1.1.3 Structure-Based Approaches to Colchicine Site Inhibitors
1.2 Molecular Modeling on Compounds Targeting the Vinca Alkaloid Binding Site
2 Molecular Modeling on Microtubule-Stabilizing Agents
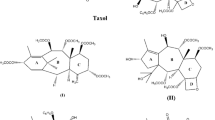
2.1 Molecular Modeling on Paclitaxel and Taxanes
2.1.1 The First Electron Crystallography Structure of α,β-Tubulin/Paclitaxel Complex
2.1.2 The Oxetane Issue
2.1.3 The Paclitaxel Binding Mode
2.2 Epothilones
2.3 Common Pharmacophores
2.4 Other Stabilizing Agents
2.4.1 Discodermolide and Dictyostatin
2.4.2 Sarcodictyins and Eleutherobins
2.4.3 Laulimalide and Peloruside
3 Conclusions
References
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- TMP:
-
trimethoxyphenyl
- CA4:
-
combretastatin A-4
- CSI:
-
colchicine site inhibitors
- DAMA-colchicine:
-
(N-deacetyl-N-(2-mercaptoacetyl)colchicine)
- EPO:
-
epothilone
- MSAA:
-
microtubule-stabilizing antimitotic agents
- MD:
-
molecular dynamics
- MIF:
-
molecular interaction field
- PTX:
-
paclitaxel
References
JONBO1. Jordan MA, Wilson L (2004) Nat Rev Cancer 4:253
Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M (2004) Nature 428:198
Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M (2005) Nature 435:519
Downing KH, Nogales E (1998) Curr Opin Cell Biol 10:16
Prinz H (2002) Exp Rev Anticancer Ther 2:695
Li Q, Sham HL (2002) Exp Opin Ther Patents 12:1663
Checchi PM, Nettles JH, Zhou J, Snyder JP, Joshi HC (2003) Trends Pharmacol Sci 24:361
Gupta S, Bhattacharyya B (2003) Mol Cell Biochem 253:41
Hastie SB (1991) Pharmacol Ther 51:377
Bhattacharyya B, Panda P, Gupta S, Banerjee M (2008) Med Res Rev 28:155
Nakagawa-Goto K, Chen CX, Hamel E, Wu CC, Bastow KF, Brossi A, Lee KH (2005) Bioorg Med Chem Lett 15:235
Beckers T, Mahboobi S (2003) Drugs Fut 28:767
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D (1989) Experientia 45:680
Pettit GR, Cragg GM, Singh SB (1987) J Nat Prod 50:386
Pettit GR, Cragg GM, Herald DL, Schmidt JM, Lohavanijaya P (1982) Can J Chem 60:1374
Lin CM, Ho HH, Pettit GR, Hamel E (1989) Biochemistry 28:6984
Jordan A, Hadfield JA, Lawrence NJ, McGown AT (1998) Med Res Rev 18:259
Nam NH (2003) Curr Med Chem 10:1697
Ohsumi K, Nakagawa R, Fukuda Y, Hatanaka T, Morinaga Y, Nihei Y, Ohishi K, Suga Y, Akiyama Y, Tsuji T (1998) J Med Chem 41:3022
Pettit GR, Temple C, Jr Narayanan VL, Varma R, Simpson MJ, Boyd MR, Rener GA, Bansal N (1995) Anticancer Drug Res 10:299
Nihei Y, Suzuki M, Okano A, Tsuji T, Akiyama Y, Tsuruo T, Saito S, Hori K, Sato Y (1999) Jpn J Cancer Res 90:1387
Wang L, Woods KW, Li Q, Barr KJ, McCroskey RW, Hannick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee JY, Zielinski-Mozng N, Frost D, Rosenberg SH, Sham HL (2002) J Med Chem 45:1697
Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E (1988) Mol Pharmacol 34:200
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA (2006) J Med Chem 49:3033
Ter Haar E, Rosekranz HS, Hamel E, Day BW (1996) Bioorg Med Chem 4:1659
Brown ML, Rieger JM, Macdonald TL (2000) Bioorg Med Chem 8:1433
Weigt M, Weise M (2000) QSAR J 19:142
Zhang SX, Feng J, Kuo SC, Brossi A, Hamel E, Tropsha A, Lee KH (2000) J Med Chem 43:167
Berg U, Bladh H (1999) Helv Chim Acta 82:323
Brossi A, Lee KH, Yeh HJC (1999) Helv Chim Acta 82:1223
Ducki S, Mackenzie G, Lawrence NJ, Snyder JP (2005) J Med Chem 48:457
Vedani A, Dobler M (2002) J Med Chem 45:2139
Feher M, Schmidt JM (2000) J Chem Inf Comput Sci 40:495
Kearsley SK, Smith GM (1990) Tetrahedron Comput Methods 3:615
De Martino G, Edler MC, La Regina G, Coluccia A, Barbera MC, Barrow D, Nicholson RI, Chiosis G, Brancale A, Hamel E, Artico M, Silvestri R (2006) J Med Chem 49:947
Rappl C, Barbier P, Bourgarel-Rey V, Grégoire C, Gilli R, Manon C, Combes S, Finet JP, Peyrot V (2006) Biochemistry 45:9210
Kong Y, Grembecka J, Edler MC, Hamel E, Mooberry SL, Sabat M, Rieger J, Brown ML (2005) Chem Biol 12:1007
Nguyen TL, McGrath C, Hermone AR, Burnett JC, Zaharevitz DW, Day BW, Wipf P, Hamel E, Gussio R (2005) J Med Chem 48:6107
Kim DY, Kim KH, Kim ND, Lee KY, Han CK, Yoon JH, Moon SK, Lee SS, Seong BL (2006) J Med Chem 49:5564
Bellina F, Cauteruccio S, Monti S, Rossi R (2006) Bioorg Med Chem Lett 16:5757
Zhang Q, Peng Y, Wang XI, Keenan SM, Arora S, Welsh WJ (2007) J Med Chem 50:749
De Martino G, La Regina G, Coluccia A, Edler MC, Barbera MC, Brancale A, Wilcox E, Hamel E, Artico M, Silvestri R (2004) J Med Chem 47:6120
La Regina G, Edler MC, Brancale A, Kandil S, Coluccia A, Piscitelli F, Hamel E, De Martino G, Matesanz R, Diaz JF, Scovassi AI, Prosperi E, Lavecchia A, Novellino E, Artico M, Silvestri R (2007) J Med Chem 50:2865
Romagnoli R, Baraldi PG, Pavani MG, Tabrizi MA, Preti D, Fruttarolo F, Piccagli L, Jung MK, Hamel E, Borgatti M, Gambari R (2006) J Med Chem 49:3906
Bai R, Covell DG, Pei XF, Ewell JB, Nguyen NY, Brossi A, Hamel E (2000) J Biol Chem 275:40443
Nogales E, Wolf SG, Downing KH (1998) Nature 391:199
Bai R, Pettit GR, Hamel E (1990) J Biol Chem 265:17141
Mitra A, Sept D (2004) Biochemistry 43:13955
Löwe J, Li H, Downing KH, Nogales E (2001) J Mol Biol 313:1045
Barbier P, Gregoire C, Devred F, Sarrazin M, Peyrot V (2001) Biochemistry 40:13510
Ojima I, Chakravarty S, Inoue T, Lin S, He L, Horwitz SB, Kuduk SD, Danishefsky SJ (1999) Proc Natl Acad Sci U S A 96:4256
Suffness M, Wall ME (1995) In: Suffness M Taxol: science and applications. CRC Press, Boca Ranton, FL, pp 3–25.
Schiff PB, Fant J, Horwitz SB (1979) Nature 277:665
Höfle G, Bedorf N, Gerth K, Reichenbach H (1993), (GBF) DE-4138042 1993; Chem Abstr
Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM (1995) Cancer Res 55:2325
Hung DT, Chen J, Schreiber SL (1996) Chem Biol 3:287
Longley RE, Gunasekera SP, Faherty D, McLane J, Dumont F (1993) Ann NY Acad Sci 696:94
Long BH, Carboni JM, Wasserman AJ, Cornell LA, Casazza AM, Jensen PR, Lindel T, Fenical W, Fairchild CR (1998) Cancer Res 58:1111–1115
Hamel E, Sackett DL, Vourloumis D, Nicolaou KC (1999) Biochemistry 38:5490
He L, Jagtap PG, Kingston DG, Shen HJ, Orr GA, Horwitz SB (2000) Biochemistry 39:3972
Wilson L, Jordan MA (2004) Chemotherapy 16:83
Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L (1996) Cancer Res 56:816
Nuijen B, Bouma M, Schellens JH, Beijnen JH (2001) Invest New Drugs 19:143
Aversa SM, Cattelan AM, Salvagno L, Crivellari G, Banna G, Trevenzoli M, Chiarion-Sileni V, Monfardini S (2005) Crit Rev Oncol Hematol 53:235
Mekhail TM, Markman M (2002) Exp Opin Pharmacother 3:755
Zhao J, Kim JE, Reed E, Li QQ (2005) Int J Oncol 27:247
Ballone P, Marchi M (1993) J Phys Chem 103:3097
Milanesio M, Ugliengo P, Viterbo D (1999) J Med Chem 42:291
Guéritte-Voegelein F, Guénard D, Mangatal K, Poitier P, Guilhem J, Césario M, Pascard C (1990) Acta Crystallogr Sect C 46:781
Jiménez-Barbero J, Amat-Guerri F, Snyder JP (2002) Curr Med Chem Anticancer Agents 2:91
Brünger AT, Karplus M (1991) Acc Chem Res 24:54
Leach AR (1996) Molecular modelling, principles and applications. Longman, London, pp 436–442
Mastropaolo D, Camerman A, Luo Y, Brayer GD, Camerman N (1995) Proc Natl Acad Sci U S A 92:6920
Vander Velde DG, Georg GI, Grunewald GL, Gunn CW, Mitscher LA (1993) J Am Chem Soc 115:11650
Williams HJ, Scott AI, Dieden RA, Swindell CS, Chirlian LE, Francl MM, Heerding JM, Krauss NE (1993) Tetrahedron 49:6545
Boge TC, Wu ZJ, Himes RH, Vander Velde DG, Georg GI (1999) Bioorg Med Chem Lett 9:3047
Dubois J, Guénard D, Guéritte-Voegelein F, Guedira N, Potier P, Gillet B, Beloeil JC (1993) Tetrahedron 49:6533
Snyder JP, Nevins N, Cicero DO, Jansen JJ (2000) Am Chem Soc 122:724
Snyder JP, Nettles JH, Cornett B, Downing KH, Nogales E (2001) Proc Natl Acad Sci U S A 98:5312
Cachau RR, Gussio R, Beutler JA, Chmurny GN, Hilton BD, Muschik GM, Erickson JW (1994) Supercomput Appl High Performance Comput 8:24
Accelrys, Inc.: San Diego, CA, 2008
Wang M, Xia X, Kim Y, Hwang D, Jansen JM, Botta M, Liotta DC, Snyder JP (1999) Org Lett 15:43–46
Zbinden P, Max D, Folkers G, Vedani A (1998) QSAR 17:112
Rao S, Krauss NE, Heerding JM, Swindell CS, Ringel I, Orr GA, Horwitz SB (1994) J Biol Chem 269:3132
Rao S, Orr GA, Chaudray AG, Kingston DGI, Horwitz SB (1995) J Biol Chem 35:20235
Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sacket D, Nicolaou KC, Fojo T (2000) Proc Natl Acad Sci U S A 6:2904
He L, Jagtap PG, Kingston DG, Shen HJ, Orr GA, Horwitz SB (2000) Biochemistry 39:3972
Barboni L, Lambertucci C, Appendino G, Vander Velde DG, Himes RH, Bombardelli E, Wang M, Snyder JP (2001) J Med Chem 44:1576
Querolle O, Dubois J, Thoret S, Roussi F, Montiel-Smith S, Guéritte F,Guénard D (2003) J Med Chem 46:3623
Ganesh T, Guza RC, Bane S, Ravindra R, Shanker N, Lakdawala AS, Snyder JP, Kingston DG (2004) Proc Natl Acad Sci U S A 101:10006
Shanker N, Kingston DG, Ganesh T, Yang C, Alcaraz AA, Geballe MT, Banerjee A, McGee D, Snyder JP, Bane S (2007) Biochemistry 46:11514
Gao Q, Wei JM, Chen SH (1995) Pharm Res 12:337
Gao Q, Chen SH (1996) Tetrahedron Lett 37:3425
Paloma LG, Guy RK, Wrasidlo W, Nicolaou KC (1994) Chem Biol 1:107
Ojima I, Kuduk SD, Chakravarty S, Ourevitch M, Bégue JP (1997) J Am Chem Soc 119:5519
Jiménez-Barbero J, Souto AA, Abal M, Barasoain I, Evangelio JA, Acuña AU, Andreu JM, Amat-Guerri F (1998) Biorg Med Chem 6:1857
Ewing TJA, Kuntz ED (1997) J Comp Chem 18:1175
Barboni L, Datta A, Dutta D, Georg G, Velde DG, Himes RH, Wang M, Snyder JP (2001) J Org Chem 66:3321
Wang M, Cornett B, Nettles J, Liotta DC, Snyder JP (2000) J Org Chem 65:1059
Gueritte F (2001) Curr Pharm Des 7:1229
Barboni L, Giarlo G, Riccutelli M, Ballini R, Georg G, VanderVelde DG, Himes RH, Wang M, Lakdawala A, Snyder JP (2004) Org Lett 6:461
Wu JH, Zamir LO (2000) Anticancer Drug Des 15:73
Nogales E, Whittaler M, Milligan RA, Downing KH (1999) Cell 96:79
Amos L, Lowe A (1999) J Chem Biol 6:R65; Buey RM, Díaz FJ, Andreu JM, O’Brate A, Giannakakou P, Nicolaou KC, Sasmal PK, Ritzén A, Namoto K (2004) Chem Biol 11:225
Nogales E (1999) Cel Mol Life Sci 56:133
Miller ML, Ojima I (2001) Chem Rec 1:195
Mastalerz H, Zhang G, Kadow J, Fairchild C, Long B, Vyas DM (2001) Org Lett 3:1613
Lee KW, Briggs JM (2001) J Comput Aided Mol Des 15:41
Nicolaou KC, Vourloumis D, Li T, Pastor J, Wissinger N, He Y, Ninkovic S, Sarabaia F, Vallberg H, Roschangar F, King NP, Finlay MRV, Giannakakou P, Verdier-Pinard P, Hamel E (1997) Angew Chem Int Ed Engl 36:2093
Nicolaou KC, Roschangar F, Vourloumis D (1998) Angew Chem Int Ed Engl 37:2014
Winkler JD, Axelsen PH (1996) Bioorg Med Chem Lett 6:2963
Chou T, Zhang X, Balog A, Su D, Meng D, Savin K, Bertino JR, Danishefsky SJ (1997) Proc Natl Acad Sci U S A 95:9642
Rao S, Krauss NE, Heerding JM, Swindell CS, Ringel I, Orr GA, Horwitz SB (1994) J Biol Chem 269:3132
Manetti F, Forli S, Maccari L, Corelli F, Botta M (2001) Il Farmaco 58:375
Manetti F, Maccari L, Corelli F, Botta M (2004) Curr Topics Med Chem 4:203
Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH (2004) Science 305:866
Carlomagno T, Blommers MJ, Meiler J, Jahnke W, Schupp T, Petersen F, Schinzer D, Altmann KH, Griesinger C (2007) Angew Chem 115:2615
Reese M, Sánchez-Pedregal VM, Kubicek K, Meiler J, Blommers MJ, Griesinger C, Carlomagno T (1997) Angew Chem Int Ed Engl 46:1864
Sánchez-Pedregal VM, Reese M, Meiler J, Blommers MJ, Griesinger C, Carlomagno T (2005) Angew Chem Intl Ed Engl 44:4172
Botta M, Forli S, Altmann K-H (unpublished results)
Bold G, Wojeik S, Caravatti G, Lindauer R, Stierlin C, Gertsch J, Wartmann M, Altmann KH (2006) ChemMedChem 1:37
Zhan W, Jiang Y, Brodie PJ, Kingston DI, Liotta DC, Snyder JP (2008) Org Lett 10:1565
Martello L, LaMarche MJ, He L, Beauchamp TJ, Smith AB, Hotwitz SB (2001) Chem Biol 8:843
Sánchez-Pedregal VM, Kubicek K, Meiler J, Lyothier I, Paterson I, Carlomagno T (2006) Angew Chem Int Ed Engl 45:7388
Smith ABI, LaMarche MJ, Falcone-Hindley M (2001) Org Lett 5:695
Petitt GR, Chichacz ZA, Gao F, Boyd MR, Schmidt JM (1994) J Chem Soc Chem Commun 1:1111
Madiraju C, Edker MC, Hamel E, Raccor BS, Balachandran R, Zhu G, Giuliano KA, Vogt A, Shin Y, Fournier JH, Fukui Y, Bruckner AM, Curran DP, Day BW (2005) Biochemistry 44:15053
Mooberry SL, Tien G Hernandez AH, Plubrukar A, Davidson BS (1999) Cancer Res 59:653
Pryor DE, O’Brate A, Bilcer G, Diaz JF, Wang Y, Wang Y, Kabaki M, Jung MK, Andreu JM, Ghosh AK, Giannakakou P, Hamel E (2002) Biochemistry 41:9109
Gapud EJ, Bai R, Ghosh AK, Hamel E (2004) Mol Pharmacol 66:113
Gaitanos TN, Buey RM, Diaz JF, Northcote PT, Teesdale-Spittle P, Andreu JM, Miller JH (2004) Cancer Res 64:5063
Miller JH, Rouwe B, Gaitanos TN, Hood KA, Crume KP, Backstrom BT, La Flamme AC, Berridge MV, Northcote PT (2004) Apoptosis 9:785
Pineda O, Farràs J, Maccari L, Manetti F, Botta M, Vilarrasa J (2004) Bioorg Med Chem Lett 14:4825
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) J Comput Chem 19:1639
Jones G, Willet P, Glen RC, Leach AR, Taylor J (1997) J Mol Biol 267:727
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Springer-Verlag London
About this chapter
Cite this chapter
Botta, M., Forli, S., Magnani, M., Manetti, F. (2008). Molecular Modeling Approaches to Study the Binding Mode on Tubulin of Microtubule Destabilizing and Stabilizing Agents. In: Carlomagno, T. (eds) Tubulin-Binding Agents. Topics in Current Chemistry, vol 286. Springer, Berlin, Heidelberg. https://doi.org/10.1007/128_2008_20
Download citation
DOI: https://doi.org/10.1007/128_2008_20
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-69036-8
Online ISBN: 978-3-540-69039-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)