Abstract
Purpose
The remarkable amount of preclinical data achieved on 89Zr-PET imaging led to a significant clinical translation, concerning mainly immuno-PET applications. The aim of this systematic review is to provide a complete overview on clinical applications of 89Zr-PET imaging, using a systematic approach to identify and collect published studies performed in humans, sorted by field of application and specific disease subsections.
Methods
A systematic literature search of articles suiting the inclusion criteria was conducted on Pubmed, Scopus, Central, and Web Of Science databases, including papers published from January 1967 to November 2020. Eligible studies had to be performed on humans through PET imaging with 89Zr-labeled compounds. The methodological quality was assessed through the Quality Assessment of Diagnostic accuracy Studies-2 tool.
Results
A total of 821 articles were screened. 74 studies performed on humans were assessed for eligibility with the exclusion of further 18, thus 56 articles were ultimately selected for the qualitative analysis.
Conclusions
89Zr has shown to be a powerful PET-imaging tool, in particular for radiolabeling antibodies in order to study antigen expression, biodistribution, anticancer treatment planning and follow-up. Other than oncologic applications, 89Zr-radiolabeled antibodies have been proposed for use in inflammatory and autoimmune disorders with interesting results. 89Zr-labeled nanoparticles represent groundbreaking radiopharmaceuticals with potential huge fields of application. To evaluate the clinical usefulness of 89Zr PET-imaging in different conditions and in real-world settings, and to widen its use in clinical practice, further translation of preclinical to clinical data is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
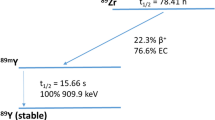
Among the large and still growing number of valuable radiotracers developed for positron emission tomography (PET) applications in precision medicine, the emerging radiometal Zirconium-89 (89Zr) has recently shown great potential in various fields of application. Produced by a medical cyclotron at a low cost, in a reasonable time and without high impurity, 89Zr decays via positron emission (23%) and electron capture (77%) to a metastable level of Yttrium (89mY), which in turn decays via γ-ray emission to stable 89Y [73]. The associated photons of 908.97 keV (99% abundance) emitted from the excited state can be separated by setting the energy windows and are not in coincidence with the PET signal, due to the half-life of the metastable level (t1/2 = 15.8 s) [11]. The relatively low-energy of 89Zr-positrons (Eβ+,max = 897 keV; Eβ+,ave = 396.9 keV) leads to a maximum positron-range in water of 3.6 mm, thus providing high-resolution PET images [71]. With a long half-life of 3.3 days, 89Zr as other long-lived radionuclides, such as 64Cu (t1/2 = 12.7 h), 76Br (t1/2 = 16.2 h), 86Y (t1/2 = 14.7 h) and 124I (t1/2 = 4.2 days), is particularly apt to follow radiopharmaceuticals for several days and up to one week[59], allowing for reliable biodistribution assessment [75]. Assuming that 89Zr shares the same residualizing properties of other known radionuclides, such as 90Y, it might be internalized and trapped after binding to the surface of cells, resulting in extremely high target to-background ratios [65], but as a metallo-radionuclide, it requires complexation to prevent non-specific binding to non-targeted tissues, particularly to the bone marrow [71]. Desferrioxamine B (DFO), an iron-sequestering natural bacterial siderophore, with hydroxamate groups as binding sites for 89Zr, represents by far the most commonly used bifunctional chelator, particularly suitable for labeling monoclonal antibodies (mAbs) [72]. mAbs have been widely used for both diagnostic and therapeutic purposes for decades in a broad range of medical indications, particularly in oncology [42]. The specific imaging features paired with the favorable matching between the long half-life and the slower pharmacokinetics of mAbs, make 89Zr an effective imaging tool for antibody or immune-based PET, referred to as “immuno-PET” [39]. Combining the sensitivity of PET with the specificity of mAbs, immuno-PET aims to provide a non-invasive and direct readout of antigen expression, along with a reliable characterization of both pharmacokinetic and dosimetric properties of mAbs [69]. The application of radiolabeled mAbs as imaging probes for both diagnosis and antibody-based treatment planning has always been a vibrant area in molecular imaging and has generated great interest in PET-labeling strategies [33, 50]. To date, immuno-PET represents by far the widest field of application of 89Zr-PET imaging, but in recent years, a growing number of studies has focused on 89Zr-labeled nanoparticles (NPs) with promising results in tumor detection, drug monitoring, inflammation imaging, as well as tumor-associated macrophage and sentinel lymph mapping [70]. The substantial preclinical data flourished on 89Zr-PET imaging have led to a successful translation to clinical trials.
This systematic review aims to provide a complete overview of clinical applications of 89Zr-PET imaging, using a systematic approach to identify and collect published studies performed in humans.
Materials and methods
Search strategy and study selection
This systematic review was drawn up according to PRISMA guidelines [31]. An online literature search of articles that suited the inclusion criteria was conducted on Pubmed, Scopus, Central (Cochrane Library), and Web Of Science databases and included papers published from January 1967 to November 2020. The following search keywords were applied for each database: “Positron Emission Tomography” OR “PET” AND “Zirconium-89” OR “89Zr”. Eligible studies had to be performed on humans through PET imaging with 89Zr-labeled compounds. The references of the provided articles were also examined in order to find out any additional relevant studies. For the inclusion in the qualitative analysis English language was mandatory.
Data extraction and methodological quality assessment
General data about the article, specifically authors, journal, year of publication, country, study design, and patient characteristics, were retrieved for all the included studies.
The methodological quality was assessed through the Quality Assessment of Diagnostic accuracy Studies-2 (QUADAS 2) tool, widely used and recommended for systematic reviews of diagnostic accuracy by the Agency for Healthcare Research and Quality, Cochrane Collaboration (Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy), and the U.K. National Institute for Health and Clinical Excellence [53, 67, 68]. Both data extraction and quality assessment were independently performed by two reviewers and eventual disagreements were resolved by unanimous approval after discussion.
Results
Search results
A total of 821 articles were screened by examining each abstract to identify potentially suitable studies. From the overall group of 821, 437 articles concerning preclinical studies, four studies performed on phantoms, 79 studies focusing on 89Zr production and/or radiolabeling chemistry, as well as 68 reviews, 141 articles not satisfying the inclusion criteria and 18 articles not in English language were excluded. The 74 remaining studies performed on humans, were assessed for eligibility with the exclusion of further 18 papers (four case reports, four dosimetric studies, three studies performed on healthy volunteers, five analyses of clinical data and two studies with no full text available). 56 articles were considered suitable and ultimately selected for the qualitative analysis of this systematic review. Search strategy and selection criteria applied are represented in flow chart (see Fig. 1).
Study characteristics
The 56 selected papers were published from 2006, year of publication of the first-in-human study with 89Zr as PET-imaging radionuclide [7], to 2020. Most studies were conducted by authors from the USA and Europe, with two studies performed by researchers from Japan. The number of enrolled patients ranged from 3 to 268, the latter corresponding with the ARTFORCE randomized phase II trial for individualized treatment of head and neck cancer [22]. As subjects were primarily cancer patients, selected studies were mostly about immuno-PET imaging in the oncological field. A minority of studies focused on immuno-PET applications other than oncological ones (n = 6), while only few studies (n = 4) were performed with 89Zr-labeled NPs.
Methodological quality assessment
Considering the methodological quality of the studies included as a whole, this was found to be quite good: 42 of 56 studies satisfied at least three and 22 of 56 all of the four QUADAS-2 domains for the bias risk assessment and 40 satisfied each of the three applicability assessment domains, with 49 satisfying at least two domains (Table 1). Pondering the results within each bias assessment domain in a independent way, at least 52 studies obtained a low concern of bias and no more than nine studies showed high risk in some of those (see Fig. 2 and Fig. 3). Taking into account all four bias assessment domains, only nine studies reported more than two unclear results, in relation to insufficient information given to achieve an optimal methodological protocol appraisal, and no one reported a high risk of bias more than one domain. Considering the patient selection domain, three studies had an unclear risk of bias because of a lack of detailed information or it was not reported patients enrollment. Two studies reported high risk of bias because of the heterogeneity of the inclusion criteria. For index test domain, two studies reported high risk of bias as a consequence of different PET imaging acquisition and/or elaboration work out, leading to a certain grade of heterogeneity. A high concern of applicability was found, both in index test domains and patient selection, in three studies. Finally, the concern for global applicability was mainly low (see Figs. 4 and 5).
Discussion
89Zr-immuno-PET
Oncological applications
Breast cancer
As treatment options with targeting agents such as trastuzumab, pertuzumab and trastuzumab-emtansine for patients with metastatic breast cancer are largely dependent upon the presence of human epidermal growth factor receptor 2 (HER2), the development of non-invasive imaging techniques for the assessment of HER2 status has generated great interest in recent years. Laforest et al. evaluated radiation dosimetry of 89Zr-trastuzumab PET, with liver being the dose-limiting organ, reported a safe profile and defined optimal imaging time at least four days post-injection [29]. Several studies investigated the role of 89Zr-trastuzumab PET imaging in selecting metastatic patients with HER2-positive lesions, with Ulaner et al. focusing on patients with HER2-negative primary breast cancer [16, 55, 56]. In 2018 Dehdashti et al. showed the potential of 89Zr-trastuzumab PET in characterizing HER2 status of patients with breast cancer, in order to avoid repeat or multiple tissue sampling [13], while Bensch et al. reported its value in supporting diagnostic understanding and clinical decision making in twenty patients with unclear HER2 status after standard workup, specifically bone scan, 18F-FDG PET, CT and, if feasible, biopsy [4]. The ZEPHIR trial performed by Gebhart et al. investigated the heterogeneity of advanced HER2-positive breast cancer and demonstrated the prognostic value of combining 18F-FDG PET and 89Zr-trastuzumab PET in predicting patient outcome under Trastuzumab-emtansine treatment [19]. In a recent work published in 2020, Ulaner et al. successfully identified HER2-positive metastases in six patients with HER2-negative primary breast cancer through 89Zr-pertuzumab PET [54], after a preliminary first-in-human dosimetric study [57]. As concerning tumor angiogenesis, in 2013 Gaykema et al. performed a feasibility study with 89Zr-bevacizumab PET investigating whether vascular endothelial growth factor (VEGF)-A imaging can be used for detection of primary breast tumors [17]. In a following paper, the same authors used both 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in sixteen patients with advanced HER2 or estrogen receptor-positive metastatic breast cancer [18].
Head and neck cancer (HNC)
In the first in humans 89Zr immuno-PET study published in 2006 by Bӧrjesson et al., the 89Zr-labeled chimeric mAb U36 showed high accuracy in the detection of both primary tumor and lymph node metastases in twenty patients head and neck squamous cell carcinoma (HNSCC) [7]. In a later study 89Zr-labeled mAb U36 was used to quantitatively assess biodistribution, uptake, organ residence times, and radiation dose in HNSCC patients planned to undergo neck dissection [8]. In a randomized controlled phase II trial, Heukelom et al. analyzed the predictive value of 89Zr-cetuximab uptake to improve patient selection for chemoradiotherapy with either the anti-epidermal growth factor receptor (EGFR) mAb cetuximab, or cisplatin [22].
Prostate cancer
In 2014 Osborne et al. published the results of a pilot study exploring the feasibility of 89Zr-PET imaging with radiolabeled J591, a humanized mAb that specifically binds to the prostate-specific membrane antigen (PSMA) extracellular domain, in eleven patients with localized prostate cancer undergoing radical prostatectomy and demonstrated the usefulness of 89Zr-J591 PET in identifying tumor foci, as well as a relationship between tumor uptake and aggressiveness, as defined by Gleason score [45]. Similar data on 89Zr-J591 PET have been reported in patients with metastatic prostate cancer (mPC) by Pandit-Taskar et al., who performed a first-in-human phase I/II study comparing the detection ability of 89Zr-J591 PET with conventional imaging modalities and pathology [47], following a preliminary work on safety, biodistribution and dosimetry [46]. Exploiting the relatively new identification of the six-transmembrane epithelial antigen of prostate-1 (STEAP1), O’Donoghue et al. assessed pharmacokinetics and biodistribution of 89Zr-DFO-MSTP2109A, a radiolabeled antibody that recognizes STEAP1 in seven patients with metastatic castration resistant prostate cancer (mCRPC) who had at least one archival STEAP1-positive biopsy [40]. In a later study published in November 2019, Carrasquillo et al. confirmed excellent tumor uptake in both soft tissue and bone lesions in nineteen mCRPC patients and proposed 89Zr-DFO-MSTP2109A as a companion diagnostic in patients undergoing STEAP1-directed therapy warranting further exploration [10]. Aiming to provide new insights on mPC imaging, Pandit-Taskar et al. conducted a first-in-human phase I dose-escalation PET-imaging study with the anti-PSMA minibody “IAB2M” labeled with 89Zr, and reported favorable pharmacokinetics, biodistribution, dosimetry, and lesion uptake of the radiopharmaceutical for targeting mPC [48].
Urological malignancies
The above-mentioned radiopharmaceutical has been subsequently used in a phase I/IIa PET imaging study among patients with urological malignancies (thirteen patients with prostate cancer and four patients with other urological cancer) by Joraku et al. who confirmed the safety and tolerability of 89Zr-IAB2M in terms of adverse events and radiation exposure and protection [26].
Gastrointestinal tumors
The mAb Trastuzumab is the first Food and Drug Administration–approved targeted agent for treatment of patients with esophagogastric adenocarcinoma (EGA) overexpressing the anti-human epidermal growth factor receptor 2 (HER2). In 2018, O’Donoghue et al. published a first report evaluating safety, pharmacokinetics, biodistribution, and dosimetry of 89Zr-trastuzumab PET as a non-invasive imaging tool for assessing HER2 expression in patients with EGA [41]. Based on these data, Sanchez-Vega et al. conducted a phase II trial of afatinib, an irreversible pan-HER kinase inhibitor, in patients with trastuzumab-refractory HER2-amplified EGA, after performing functional imaging with 89Zr-trastuzumab PET to determine sensitivity and resistance to therapy [52]. In a feasibility study, Moek et al. assessed the biodistribution of AMG 211, a bispecific T-cell engager against carcinoembryonic antigen on tumor cells and cluster of differentiation 3 (CD3) on T-cells, by radiolabeling AMG 211 with 89Zr in patients with advanced gastrointestinal adenocarcinomas [37]. The anti-epidermal growth factor receptor (EGFR) mAbs cetuximab and panitumumab have been radiolabeled with 89Zr for PET imaging in order to investigate biodistribution, tumor uptake and response to treatment in patients with advanced colorectal cancer. Van der Houven van Oordt et al. proposed 89Zr-cetuximab PET/CT as a potential selection method for cetuximab treatment needing further clinical validation [35], while van Helden et al. failed to predict treatment benefit and progression free survival (PFS) through 89Zr-PET imaging, underlining the different factors which may correlate with intrinsic resistance to therapy [63]. In 2017, Lindenberg et al. reported the first in human experience and study of dosimetry with 89Zr-panitumumab [32].
Renal cell carcinoma (RCC)
To find predictive biomarkers for antiangiogenic treatment, 89Zr-bevacizumab has been investigated as vascular endothelial growth factor A (VEGF-A)-binding PET-tracer in metastatic RCC (mRCC) patients. Following a pilot study revealing a significant decrease of 89Zr-bevacizumab uptake during treatment with bevacizumab/interferon-α and sunitinib [43], van Es et al. reported similar findings with everolimus in thirteen patients with mRCC and prospected a potential role of 89Zr-bevacizumab PET in selecting patients for everolimus treatment [62].
Exploiting the high expression of carbonic anhydrase IX (CAIX) in clear cell RCC (ccRCC), Hekman et al. showed the value of PET/CT imaging with the 89Zr-labeled anti-CAIX mAb girentuximab in case of diagnostic dilemmas concerning ccRCC [21], while Verhoeff et al. underlined how the addition of 89Zr-girentuximab-PET/CT and 18F-FDG-PET/CT increases lesion detection compared to CT alone in newly diagnosed metastatic ccRCC (mccRCC) patients [66].
Brain tumors
Using 89Zr-Df-IAB2M (anti-PSMA minibody) PET scans, Matsuda et al. showed high expression of PSMA in tumor-associated neovasculature of high-grade gliomas and metastatic brain tumors in respect to primary central nervous system lymphomas (PCNSL) and radiation necrosis, and prospected a potential role of 89Zr-PET imaging in the differential diagnosis and prediction of treatment response to bevacizumab therapy in high-grade gliomas [34]. Similarly, Jansen et al. proposed 89Zr-PET scanning for the selection of patients with the greatest chance of benefit from bevacizumab treatment by measuring the tumor uptake of 89Zr-labeled bevacizumab in seven children with diffuse intrinsic pontine glioma (DIPG) [25]. In a recent publication den Hollander et al. imaged transforming growth factor–b (TGF-b) expression in twelve patients with recurrent high-grade glioma using 89Zr-fresolimumab PET, but revealed no treatment benefit after fresolimumab [14].
Haematological malignancies
89Zr has been recently used for biodistribution and radiation dosimetry studies in patients with relapsed and refractory non-Hodgkin’s lymphoma (NHL) eligible for radioimmunotherapy (RIT) with 90Y-labeled anti-CD20 mAbs. Rizvi et al. demonstrated that the biodistribution of 89Zr-ibritumomab tiuxetan is not influenced by the simultaneous therapy with 90Y-ibritumomab tiuxetan in seven patients with relapsed or refractory aggressive CD20-positive B-cell NHL [51]. In 2015, Muylle et al. showed that the administration of a standard preload of unlabeled rituximab impairs tumor targeting of the radioconjugate in the majority of patients eligible for RIT, using 89Zr-rituximab PET/CT imaging in five patients with CD20-positive B-cell lymphoma and progressive disease [38]. Following successful preclinical data, Ulaner et al. managed to measure osseous disease burden in ten patients with multiple myeloma using CD38-targeted immunologic PET imaging with 89Zr-DFO-daratumumab [58].
Neuroendocrine tumors (NETs)
In a recent study van Asselt et al. investigated the effect of everolimus on tumor uptake of 89Zr-labeled bevacizumab in fourteen patients with advanced progressive well-differentiated NETs. The Authors revealed a reduction of 89Zr-bevacizumab tumor uptake during everolimus treatment and proposed serial 89Zr-bevacizumab PET as early predictive biomarker of anti-VEGF-directed treatment [60].
Lung cancer
In a pilot study, Bahce et al. visualized and quantified the uptake of bevacizumab, a monoclonal antibody against vascular endothelial growth factor-A (VEGF-A), through 89Zr-PET imaging in patients with advanced non-small cell lung cancer (NSCLC) and underlined the need for further investigation to validate this technique as a predictive biomarker for selecting patients for bevacizumab treatment [3].
Head and neck (HNC) and lung cancer
A phase I trial with 89Zr-cetuximab, a mAb specifically blocking the EGFR, was performed by van Loon et al. to determine safety and tumor uptake in three HNC patients and six NSCLC patients [64].
Pancreatic and ovarian cancer
In 2016, Lamberts et al. performed an 89Zr-PET imaging study with the Mesothelin (MSLN) antibody “MMOT0530A” in conjunction with a phase I study with the antibody–drug conjugate “DMOT4039A”, containing MMOT0530A bound to the potent mitotic agent monomethyl auristatin “MMAE” in eleven patients, seven with pancreatic and four with ovarian cancer. The authors concluded that the use of 89Zr-MMOT0530A in future trials with DMOT4039A may be used to guide individualized antibody-based treatment [30].
Solid malignancies
In 2017, Bensch et al. evaluated 89Zr-lumretuzumab uptake before and during treatment with lumretuzumab, a human epidermal growth factor receptor 3 (HER3)-targeting mAb in twenty patients with histologically confirmed locally advanced or metastatic HER3-expressing solid tumors [5]. In a later study the authors demonstrated the feasibility of PET-imaging with 89Zr-atezolizumab as a non-invasive approach to assess clinical response to programmed cell death ligand-1 (PD-L1) blockade in twenty-two patients across three tumor types (locally advanced or metastatic bladder cancer, NSCLC, or triple-negative breast cancer (TNBC) [6]. Aiming to provide new insights into the mechanisms of immunotherapy, Pandit-Taskar et al. successfully imaged tumor-infiltrating CD8-positive (CD8 +) T lymphocytes through 89Zr-IAB22M2C, a radiolabeled minibody against CD8 + T cells in six patients with solid malignancies [49]. In 2018, van Brummelen et al. conducted an immuno-PET imaging study to assess biodistribution and tumor accumulation of 89Zr-labeled cergutuzumab amunaleukin (CEA-IL2v), an immunocytokine directed against carcinoembryonic antigen (CEA), in twenty-four patients with advanced solid tumors, confirming selective and targeted tumor accumulation of this novel immunocytokine [61]. Similarly, Menke-van der Houven van Oordt et al. investigated biodistribution and tumor uptake of the 89Zr-labeled anti–HER3, mAb GSK2849330, and demonstrated a dose-dependent inhibition of tumor uptake by unlabeled mAb confirming target engagement to the HER3 receptor [36].
Non-oncologic applications
Rheumatoid Arthritis (RA)
In a recent work Bruijnen et al. demonstrated the clinical value of baseline non-invasive B-cell imaging with 89Zr-rituximab PET/CT in selecting RA responders to rituximab treatment and prospected a potential use in other B-cell drove autoimmune diseases, from systemic lupus erythematosus to Sjögren’s disease [9].
Multiple sclerosis (MS)
A pilot study was performed by Hagens et al. to assess whether 89Zr-rituximab is able to detect active CD20-positive lesions in three patients with active relapsing–remitting MS. In the two patients completing the entire study protocol, no evidence for cerebral penetration of 89Zr-rituximab was found [20].
Interstitial pneumonitis
In 2019, Adams et al. investigated the possibility of imaging 89Zr-rituximab uptake as future early predictor of treatment response in a pilot study performed in ten patients with therapy refractory interstitial pneumonitis [1].
Orbital inflammatory disease
Twelve patients with thyroid eye disease and suspected idiopathic orbital inflammation were scanned by Laban et al. to assess the potential value of 89Zr-rituximab PET/CT for diagnostic and therapeutic management of refractory inflammation [28].
Von Hippel-Lindau disease (VHL)
Twenty-two VHL patients with at least one measurable hemangioblastoma were enrolled by Oosting et al. to assess vascular endothelial growth factor A (VEGF-A) expression through 89Zr-bevacizumab PET. The Authors explored the possibility of differentiating progressive from non-progressive lesions and proposed 89Zr-bevacizumab PET as a possible tool for selecting VHL patients for anti-VEGF therapy [44].
Percutaneous biopsy
Cornelis et al. demonstrated the feasibility of using 89Zr-labeled radiotracers for PET/CT-guided biopsy in patients with metastatic prostate or breast carcinoma. Within-suite PET/CT biopsy has successfully performed a mean of 6.2 days after diagnostic imaging with 89Zr-labeled anti-PSMA (J591 antibody or minibody) or trastuzumab in seven men and five women, respectively, without the need for reinjection [12].
89Zr-labeled nanoparticles
Lymphoscintigraphy
A pilot study on the clinical feasibility of PET/CT lymphoscintigraphy with 89Zr-nanocolloidal albumin for sentinel nodes (SNs) identification was performed by Heuveling et al. in five patients with oral cavity cancer, in 2012. A head-to-head comparison with γ-camera-based imaging using 99mTc-nanocolloidal albumin showed identical drainage patterns but PET/CT lymphoscintigraphy with 89Zr-nanocolloidal albumin detected additional foci near the primary tumor site and improved the visualization of lymphatic vessels, thus resulting in more detailed anatomic localization [24]. A subsequent study performed in 2018, proved the feasibility of intraoperative detection of SNs containing 89Zr-nanocolloidal albumin using a handheld high-energy gamma probe in five patients with oral cavity cancer planned for surgical resection [23]. Similar results were reported by Ankersmit et al. using preoperative endoscopical injection of 89Zr-nanocolloidal albumin and intraoperative injection of the near-infrared (NIR) tracer Indocyanine Green (ICG) in ten patients with early colon cancer eligible for laparoscopic resection [2].
Atherosclerosis
In 2016, Zheng et al. used 89Zr for radiolabeling CER-001, an engineered lipoprotein complex mimicking pre-beta High Density Lipoproteins (HDL), and evaluated the targeted delivery of the radiopharmaceutical in atherosclerotic carotid artery plaques of eight patients. Using serial PET/CT imaging, the authors provided evidence of targeted accumulation of 89Zr-labeled CER-001 in advanced atherosclerotic plaques after infusion, thus prospecting a potential role as nano-carrier for both imaging and therapeutic purposes in future plaque-targeted delivery strategies [74].
Drawbacks and limitations
Considering the large number of ongoing studies and the perspective of an even larger clinical application, a mention of some major limitations and drawbacks of 89Zr -based radiopharmaceuticals is needed.
With regard to 89Zr production, it is worth highlighting that a medium or high-energy cyclotron and a solid target system are currently needed, limiting its use to a small number of institutes worldwide. However, in recent years some improvements in the yttrium target design, such as the use of a liquid target, showed the opportunity to facilitate 89Zr production procedure, in adequate amounts for clinical applications. Moreover, one of the major concerns regarding the clinical application of a longer-lived radionuclide is the substantial radiation dose received by patients compared to shorter-lived nuclides. In fact, considering the physical characteristics of 89Zr mentioned in the Introduction, radiation dosimetry of 89Zr-labeled mAb PET is not particularly favorable, being generally poorer as compared to the most commonly applied 18F -FDG PET, ranging approximately between 20 and 40 mSv for 37–74 MBq 89Zr (68). Novel strategies aiming to reduce radiation dose and improve radiodosimetric profile are currently under investigation. The improvements in PET technology, such as the development of digital PET systems, characterized by a higher sensitivity, may lead to a significant reduction of administered activities in clinical applications. In addition, as it is known that the instability of 89Zr-DFO chelation generates several dechelated forms of 89Zr, such as 89Zr chloride, 89Zr-citrate, and 89Zr-oxalate, which accumulates in bone marrow, recent radiochemistry studies are exploiting new chelating approaches, focusing on the development of more stable 89Zr chelating agents [15, 27].
The prevailing opinion is that the advantage of 89Zr-labeled radiopharmaceuticals to provide reliable biodistribution studies, particularly in the case of slow pharmacokinetics, is worth the above mentioned limitations.
Conclusions
Following the promising preclinical data, 89Zr has been successfully used in several clinical studies. The favorable imaging properties combined with the long half-life, make 89Zr a versatile PET-imaging tool, particularly for radiolabeling mAbs to assess antigen expression, analyze biodistribution, plan treatment and evaluate response to anticancer therapies. Despite oncology represents by far the largest field of application, 89Zr-radiolabeled mAbs have been proposed for possible use in inflammatory and autoimmune disorders with interesting results. As concerning applications other than immuno-PET, 89Zr-labeled nanoparticles represent promising radiopharmaceuticals with potential huge fields of application, ranging from lymphoscintigraphy with nanocolloidal albumin to engineered nanoparticles. Further translation of preclinical data in clinical studies is needed to assess the utility of 89Zr as PET-imaging agent in different settings and widen its use in clinical practice.
References
Adams H, Van De Garde EM, Van Moorsel CH et al (2019) [(89)Zr]Zr-rituximab PET/CT activity in patients with therapy refractory interstitial pneumonitis: a feasibility study. Am J Nucl Med Mol Imaging 9:296–308
Ankersmit M, Hoekstra OS, Van Lingen A et al (2019) Perioperative PET/CT lymphoscintigraphy and fluorescent real-time imaging for sentinel lymph node mapping in early staged colon cancer. Eur J Nucl Med Mol Imaging 46:1495–1505
Bahce I, Huisman MC, Verwer EE et al (2014) Pilot study of (89)Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res 4:35
Bensch F, Brouwers AH, Lub-De Hooge MN et al (2018) (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging 45:2300–2306
Bensch F, Lamberts LE, Smeenk MM et al (2017) (89)Zr-Lumretuzumab PET Imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin Cancer Res: Off J Am Assoc Cancer Res 23:6128–6137
Bensch F, Van Der Veen EL, Lub-De Hooge MN et al (2018) (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 24:1852–1858
Börjesson PK, Jauw YW, Boellaard R et al (2006) Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res 12:2133–2140
Börjesson PK, Jauw YW, De Bree R et al (2009) Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J Nucl Med 50:1828–1836
Bruijnen S, Tsang ASM, Raterman H et al (2016) B-cell imaging with zirconium-89 labelled rituximab PET-CT at baseline is associated with therapeutic response 24 weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res Ther 18:266
Carrasquillo JA, Fine BM, Pandit-Taskar N et al (2019) Imaging patients with metastatic castration-resistant prostate cancer using (89)Zr-DFO-MSTP2109A anti-STEAP1 antibody. J Nucl Med 60:1517–1523
Conti M, Eriksson L (2016) Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys 3:8
Cornelis FH, Durack JC, Pandit-Taskar N et al (2018) Long-half-life (89)zr-labeled radiotracers can guide percutaneous biopsy within the PET/CT suite without reinjection of radiotracer. J Nucl Med 59:399–402
Dehdashti F, Wu N, Bose R et al (2018) Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res Treat 169:523–530
Den Hollander MW, Bensch F, Glaudemans AW et al (2015) TGF-β antibody uptake in recurrent high-grade glioma imaged with 89Zr-fresolimumab PET. J Nucl Med 56:1310–1314
Deri MA, Zeglis BM, Francesconi LC et al (2013) PET imaging with 89Zr: from radiochemistry to the clinic. Nucl Med Biol 40:3–14
Dijkers EC, Oude Munnink TH, Kosterink JG et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Gaykema SB, Brouwers AH, Lub-De Hooge MN et al (2013) 89Zr-bevacizumab PET imaging in primary breast cancer. J Nucl Med 54:1014–1018
Gaykema SB, Schröder CP, Vitfell-Rasmussen J et al (2014) 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res: Off J Am Assoc Cancer Res 20:3945–3954
Gebhart G, Lamberts LE, Wimana Z et al (2016) Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol: Off J Eur Soc Med Oncol 27:619–624
Hagens MH, Killestein J, Yaqub MM et al (2018) Cerebral rituximab uptake in multiple sclerosis: A (89)Zr-immunoPET pilot study. Mult Scler 24:543–545
Hekman MCH, Rijpkema M, Aarntzen EH et al (2018) Positron emission tomography/computed tomography with (89)Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur Urol 74:257–260
Heukelom J, Hamming O, Bartelink H et al (2013) Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer 13:84
Heuveling DA, Karagozoglu KH, Van Lingen A et al (2018) Feasibility of intraoperative detection of sentinel lymph nodes with 89-zirconium-labelled nanocolloidal albumin PET-CT and a handheld high-energy gamma probe. EJNMMI Res 8:15
Heuveling DA, Van Schie A, Vugts DJ et al (2013) Pilot study on the feasibility of PET/CT lymphoscintigraphy with 89Zr-nanocolloidal albumin for sentinel node identification in oral cancer patients. J Nucl Med 54:585–589
Jansen MH, Veldhuijzen Van Zanten SEM, Van Vuurden DG et al (2017) Molecular drug imaging: (89)Zr-bevacizumab PET in children with diffuse intrinsic pontine glioma. J Nucl Med 58:711–716
Joraku A, Hatano K, Kawai K et al (2019) Phase I/IIa PET imaging study with (89)zirconium labeled anti-PSMA minibody for urological malignancies. Ann Nucl Med 33:119–127
La MT, Tran VH, Kim HK (2019) Progress of coordination and utilization of zirconium-89 for positron emission tomography (PET) studies. Nucl Med Mol Imaging 53:115–124
Laban KG, Kalmann R, Leguit RJ et al (2019) Zirconium-89-labelled rituximab PET-CT in orbital inflammatory disease. EJNMMI Res 9:69
Laforest R, Lapi SE, Oyama R et al (2016) [(89)Zr]Trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol 18:952–959
Lamberts LE, Der Houven M-V, Van Oordt CW, Ter Weele EJ et al (2016) ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin Cancer Res: Off J Am Assoc Cancer Res 22:1642–1652
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Lindenberg L, Adler S, Turkbey IB et al (2017) Dosimetry and first human experience with (89)Zr-panitumumab. Am J Nucl Med Mol Imaging 7:195–203
Lövqvist A, Humm JL, Sheikh A et al (2001) PET imaging of (86)Y-labeled anti-Lewis Y monoclonal antibodies in a nude mouse model: comparison between (86)Y and (111)In radiolabels. J Nucl Med 42:1281–1287
Matsuda M, Ishikawa E, Yamamoto T et al (2018) Potential use of prostate specific membrane antigen (PSMA) for detecting the tumor neovasculature of brain tumors by PET imaging with (89)Zr-Df-IAB2M anti-PSMA minibody. J Neurooncol 138:581–589
Der Houven M-V, Van Oordt CW, Gootjes EC, Huisman MC et al (2015) 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 6:30384–30393
Der Houven M-V, Van Oordt CW, Mcgeoch A, Bergstrom M et al (2019) Immuno-PET imaging to assess target engagement: experience from (89)Zr-anti-HER3 mAb (GSK2849330) in patients with solid tumors. J Nucl Med 60:902–909
Moek KL, Waaijer SJH, Kok IC et al (2019) (89)Zr-labeled bispecific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, heterogeneous tumor uptake. Clin Cancer Res: Off J Am Assoc Cancer Res 25:3517–3527
Muylle K, Flamen P, Vugts DJ et al (2015) Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. Eur J Nucl Med Mol Imaging 42:1304–1314
Nayak TK, Brechbiel MW (2009) Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges. Bioconjug Chem 20:825–841
O’Donoghue JA, Danila DC, Pandit-Taskar N et al (2019) Pharmacokinetics and biodistribution of a [(89)Zr]Zr-DFO-MSTP2109A Anti-STEAP1 antibody in metastatic castration-resistant prostate cancer patients. Mol Pharm 16:3083–3090
O’Donoghue JA, Lewis JS, Pandit-Taskar N et al (2018) Pharmacokinetics, biodistribution, and radiation dosimetry for (89)zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med 59:161–166
Oldham RK, Dillman RO (2008) Monoclonal antibodies in cancer therapy: 25 years of progress. J Clinical Oncol: Off J Am Soc Clin Oncol 26:1774–1777
Oosting SF, Brouwers AH, Van Es SC et al (2015) 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J Nucl Med 56:63–69
Oosting SF, Van Asselt SJ, Brouwers AH et al (2016) 89Zr-bevacizumab PET visualizes disease manifestations in patients with von hippel-lindau disease. J Nucl Med 57:1244–1250
Osborne JR, Green DA, Spratt DE et al (2014) A prospective pilot study of (89)Zr-J591/prostate specific membrane antigen positron emission tomography in men with localized prostate cancer undergoing radical prostatectomy. J Urol 191:1439–1445
Pandit-Taskar N, O’donoghue JA, Beylergil V et al (2014) 89Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging 41:2093–2105
Pandit-Taskar N, O’donoghue JA, Durack JC et al (2015) A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res: Off J Am Assoc Cancer Research 21:5277–5285
Pandit-TaskarO’donoghue NJA, Ruan S et al (2016) First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med 57:1858–1864
Pandit-Taskar N, Postow MA, Hellmann MD et al (2020) First-in-humans imaging with (89)Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med 61:512–519
Reichert JM (2008) Monoclonal antibodies as innovative therapeutics. Curr Pharm Biotechnol 9:423–430
Rizvi SN, Visser OJ, Vosjan MJ et al (2012) Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur J Nucl Med Mol Imaging 39:512–520
Sanchez-Vega F, Hechtman JF, Castel P et al (2019) EGFR and MET amplifications determine response to HER2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov 9:199–209
Schueler S, Schuetz GM, Dewey M (2012) The revised QUADAS-2 tool. Ann Intern Med 156:323
Ulaner GA, Carrasquillo JA, Riedl CC et al (2020) Identification of HER2-positive metastases in patients with HER2-negative primary breast cancer by using HER2-targeted (89)Zr-pertuzumab PET/CT. Radiology 296:370–378
Ulaner GA, Hyman DM, Lyashchenko SK et al (2017) 89Zr-trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin Nucl Med 42:912–917
Ulaner GA, Hyman DM, Ross DS et al (2016) Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer Using 89Zr-trastuzumab PET/CT. J Nucl Med 57:1523–1528
Ulaner GA, Lyashchenko SK, Riedl C et al (2018) First-in-human human epidermal growth factor receptor 2-targeted imaging using (89)Zr-pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med 59:900–906
Ulaner GA, Sobol NB, O’donoghue JA et al (2020) CD38-targeted immuno-PET of multiple myeloma: from xenograft models to first-in-human imaging. Radiology 295:606–615
Vallabhajosula S, Solnes L, Vallabhajosula B (2011) A broad overview of positron emission tomography radiopharmaceuticals and clinical applications: what is new? Semin Nucl Med 41:246–264
Van Asselt SJ, Oosting SF, Brouwers AH et al (2014) Everolimus reduces (89)Zr-bevacizumab tumor uptake in patients with neuroendocrine tumors. J Nucl Med 55:1087–1092
Van Brummelen EMJ, Huisman MC, Veen D-V, LJ, et al (2018) (89)Zr-labeled CEA-targeted IL-2 variant immunocytokine in patients with solid tumors: CEA-mediated tumor accumulation and role of IL-2 receptor-binding. Oncotarget 9:24737–24749
Van Es SC, Brouwers AH, Mahesh SVK et al (2017) (89)Zr-Bevacizumab PET: potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. J Nucl Med 58:905–910
Van Helden EJ, Elias SG, Gerritse SL et al (2020) Correction to: [89Zr]Zr-cetuximab PET/CT as biomarker for cetuximab monotherapy in patients with RAS wild-type advanced colorectal cancer. Eur J Nucl Med Mol Imaging 47:2481
Van Loon J, Even AJG, Aerts H et al (2017) PET imaging of zirconium-89 labelled cetuximab: a phase I trial in patients with head and neck and lung cancer. Radiother Oncol 122:267–273
Verel I, Visser GW, Boerman OC et al (2003) Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother Radiopharm 18:655–661
Verhoeff SR, Van Es SC, Boon E et al (2019) Lesion detection by [(89)Zr]Zr-DFO-girentuximab and [(18)F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur J Nucl Med Mol Imaging 46:1931–1939
Whiting P, Rutjes AW, Reitsma JB et al (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Winter G, Harris WJ (1993) Humanized antibodies. Immunol Today 14:243–246
Yoon JK, Park BN, Ryu EK et al (2020) Current perspectives on (89)Zr-PET imaging. Int J Mol Sci 21(12):4309
Zeglis BM, Houghton JL, Evans MJ et al (2014) Underscoring the influence of inorganic chemistry on nuclear imaging with radiometals. Inorg Chem 53:1880–1899
Zeglis BM, Lewis JS (2015) The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J Vis Exp. https://doi.org/10.3791/52521
Zhang Y, Hong H, Cai W (2011) PET tracers based on Zirconium-89. Curr Radiopharm 4:131–139
Zheng KH, Van Der Valk FM, Smits LP et al (2016) HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis 251:381–388
Zhou Y, Baidoo KE, Brechbiel MW (2013) Mapping biological behaviors by application of longer-lived positron emitting radionuclides. Adv Drug Deliv Rev 65:1098–1111
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. No funding has been received for this paper.
Author information
Authors and Affiliations
Contributions
MSDF: Literature search, literature review, data extraction and manuscript writing. MP: Literature search, literature review, quality assessment and manuscript writing. VF: Manuscript writing, editing and content planning. FC: Literature search, data extraction and quality assessment. FDC: Editing and content planning. GDV: Editing and content planning.
Corresponding author
Ethics declarations
Conflict of interest
Maria Silvia De Feo, Mariano Pontico, Viviana Frantellizzi, Ferdinando Corica, Flaminia De Cristofaro and Giuseppe De Vincentis declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Feo, M.S., Pontico, M., Frantellizzi, V. et al. 89Zr-PET imaging in humans: a systematic review. Clin Transl Imaging 10, 23–36 (2022). https://doi.org/10.1007/s40336-021-00462-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-021-00462-9