Abstract
Purpose of Review
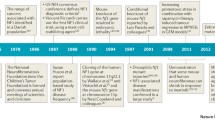
The Ras/mitogen-activated protein kinase (MAPK) pathway is essential in the regulation of cell cycle, differentiation, growth, cell senescence and apoptosis, all of which are critical to normal development. A class of neurodevelopmental disorders, RASopathies, is caused by germline mutations in genes of the Ras/MAPK pathway. Through the use of whole exome sequencing and targeted sequencing of selected genes in cohorts of panel-negative RASopathy patients, several new genes have been identified.
Recent Findings
New genes have been identified and include RIT1, SOS2, RASA2, RRAS and SYNGAP1, that likely represent new, albeit rare, causative RASopathy genes. In addition, A2ML1, LZTR1, MYST4, SPRY1 and MAP3K8 may represent new rare genes for RASopathies, but, additional functional studies regarding the mutations are warranted. In addition, recent reports have demonstrated that chromosomal copy number variation in regions encompassing Ras/MAPK pathway genes may be a novel pathogenetic mechanism expanding the RASopathies.
Summary
The identification of potential new genes and chromosomal copy number variation being associated with the RASopathies is very exciting and broadens our understanding of the biology of Ras signaling and the RASopathies.
Similar content being viewed by others
References
Papers of particular interest, published recently are highlighted as: • Of importance
• Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet. 2013;14:355–69. This is a current review of the RASopathies including phenotypic features and genetics.
Wallace MR, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249(4965):181–6.
Cawthon RM, et al. Identification and characterization of transcripts from the neurofibromatosis 1 region: the sequence and genomic structure of EVI2 and mapping of other transcripts. Genomics. 1990;7(4):555–65.
Viskochil D, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62(1):187–92.
Tartaglia M, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–8.
Roberts AE, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39(1):70–4.
Tartaglia M, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39(1):75–9.
Razzaque MA, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39(8):1013–7.
Pandit B, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39(8):1007–12.
Schubbert S, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38(3):331–6.
Cirstea IC, et al. A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat Genet. 2010;42(1):27–9.
Cordeddu V, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41(9):1022–6.
Niemeyer CM, et al. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42(9):794–800.
Martinelli S, et al. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet. 2010;87(2):250–7.
Digilio MC, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71(2):389–94.
Brems H, et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1-like phenotype. Nat Genet. 2007;39(9):1120–6.
Aoki Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–40.
Niihori T, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38(3):294–6.
Rodriguez-Viciana P, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311(5765):1287–90.
Eerola I, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73(6):1240–9.
Rajalingam K, et al. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773(8):1177–95.
Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21–44.
Marin TM, et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011;121(3):1026–43.
Chen PC, et al. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome-associated Sos1 mutation. J Clin Invest. 2010;120(12):4353–65.
Aoki Y, et al. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet. 2013;93(1):173–80.
Rusyn EV, et al. Rit, a non-lipid-modified Ras-related protein, transforms NIH3T3 cells without activating the ERK, JNK, p38 MAPK or PI3K/Akt pathways. Oncogene. 2000;19(41):4685–94.
Bertola DR, et al. Further evidence of the importance of RIT1 in Noonan syndrome. Am J Med Genet A. 2014;164A(11):2952–7.
Gos M, et al. Contribution of RIT1 mutations to the pathogenesis of Noonan syndrome: four new cases and further evidence of heterogeneity. Am J Med Genet A. 2014;164A(9):2310–6.
• Chen PC, et al. Next-generation sequencing identifies rare variants associated with Noonan syndrome. Proc Natl Acad Sci USA. 2014;111(31):11473–8. This is a very comprehensive study using whole-exome-sequencing and gene targeted sequencing to discover new genes associated with the RASopathies.
Shi GX, Andres DA. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol Cell Biol. 2005;25(2):830–46.
Yamamoto GL, et al. Rare variants in SOS2 and LZTR1 are associated with Noonan syndrome. J Med Genet. 2015;52(6):413–21.
Cordeddu V, et al. Activating mutations affecting the Dbl homology domain of SOS2 cause Noonan syndrome. Hum Mutat. 2015;36(11):1080–7.
Viskochil D. Genetics of neurofibromatosis 1 and the NF1 gene. J Child Neurol. 2002;17(8):562–70 discussion 571–2, 646–51.
Arafeh R, et al. Recurrent inactivating RASA2 mutations in melanoma. Nat Genet. 2015;47(12):1408–10.
Flex E, et al. Activating mutations in RRAS underlie a phenotype within the RASopathy spectrum and contribute to leukaemogenesis. Hum Mol Genet. 2014;23(16):4315–27.
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–74.
• Jeyabalan N, Clement JP. SYNGAP1: mind the gap. Front Cell Neurosci. 2016;10:32. This is a current through review on the RasGAP protein SynGAP.
Hamdan FF, et al. Mutations in SYNGAP1 in autosomal nonsyndromic mental retardation. N Engl J Med. 2009;360(6):599–605.
Hamdan FF, et al. De novo SYNGAP1 mutations in nonsyndromic intellectual disability and autism. Biol Psychiatry. 2011;69(9):898–901.
Komiyama NH, et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22(22):9721–32.
Muhia M, et al. Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. Eur J Neurosci. 2010;31(3):529–43.
Vissers LE, et al. Heterozygous germline mutations in A2ML1 are associated with a disorder clinically related to Noonan syndrome. Eur J Hum Genet. 2015;23(3):317–24.
Galliano MF, et al. A novel protease inhibitor of the alpha2-macroglobulin family expressed in the human epidermis. J Biol Chem. 2006;281(9):5780–9.
Schepens I, et al. The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS One. 2010;5(8):e12250.
van Trier DC, et al. External ear anomalies and hearing impairment in Noonan Syndrome. Int J Pediatr Otorhinolaryngol. 2015;79(6):874–8.
Barnes H, et al. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J Biol Chem. 2001;276(22):19119–25.
Craig J, et al. The LDL receptor-related protein 1 (LRP1) regulates the PDGF signaling pathway by binding the protein phosphatase SHP-2 and modulating SHP-2- mediated PDGF signaling events. PLoS One. 2013;8(7):e70432.
Nacak TG, et al. The BTB-kelch protein LZTR-1 is a novel Golgi protein that is degraded upon induction of apoptosis. J Biol Chem. 2006;281(8):5065–71.
Dhanoa BS, et al. Update on the Kelch-like (KLHL) gene family. Hum Genomics. 2013;7:13.
Piotrowski A, et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014;46(2):182–7.
Kraft M, et al. Disruption of the histone acetyltransferase MYST4 leads to a Noonan syndrome-like phenotype and hyperactivated MAPK signaling in humans and mice. J Clin Invest. 2011;121(9):3479–91.
Clark AM, et al. Mutational activation of the MAP3K8 protooncogene in lung cancer. Genes Chromosomes Cancer. 2004;41(2):99–108.
Shchelochkov OA, et al. Duplication of chromosome band 12q24.11q24.23 results in apparent Noonan syndrome. Am J Med Genet A. 2008;146A(8):1042–8.
Graham JM Jr, et al. Genomic duplication of PTPN11 is an uncommon cause of Noonan syndrome. Am J Med Genet A. 2009;149A(10):2122–8.
Geckinli BB, et al. Clinical report of a patient with de novo trisomy 12q23.1q24.33. Genet Couns. 2015;26(4):393–400.
Chen JL, et al. Rare copy number variations containing genes involved in RASopathies: deletion of SHOC2 and duplication of PTPN11. Mol Cytogenet. 2014;7:28.
Luo C, et al. Microduplication of 3p25.2 encompassing RAF1 associated with congenital heart disease suggestive of Noonan syndrome. Am J Med Genet A. 2012;158A(8):1918–23.
Lissewski C, et al. Copy number variants including RAS pathway genes-How much RASopathy is in the phenotype? Am J Med Genet A. 2015;167A(11):2685–90.
Yu S, Graf WD. BRAF gene deletion broadens the clinical spectrum neuro-cardio-facial-cutaneous syndromes. J Child Neurol. 2011;26(12):1593–6.
Nowaczyk MJ, et al. Deletion of MAP2K2/MEK2: a novel mechanism for a RASopathy? Clin Genet. 2014;85(2):138–46.
Risheg H, et al. Clinical comparison of overlapping deletions of 19p13.3. Am J Med Genet A. 2013;161A(5):1110–6.
• Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. This paper provides guidelines that will help standardize the criteria for the assignment of a new gene variant as being causative for a given disorder.
Acknowledgments
The authors thank patients and families for their ongoing support of research in Genomic Medicine. This work was supported in part by NIH Grant HD048502 (K.A.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
William E. Tidyman and Katherine A. Rauen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Clinical Genetics.
Rights and permissions
About this article
Cite this article
Tidyman, W.E., Rauen, K.A. Expansion of the RASopathies. Curr Genet Med Rep 4, 57–64 (2016). https://doi.org/10.1007/s40142-016-0100-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40142-016-0100-7