Abstract
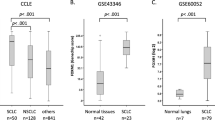
Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ, also known as WWTR1) are core downstream effectors of the Hippo pathway, which is involved in diverse biological processes. The oncogenic effects of YAP and TAZ in non-small cell lung cancer (NSCLC) have recently been reported; however, their roles in SCLC remain unclear. Immunohistochemistry (IHC) on lung cancer tissues and Western blotting (WB) on lung cancer cell lines were performed to examine the expression of YAP1. Genome editing using CRISPR/Cas9 was then used to knockout the YAP1 gene in the H69AR cell line. An RNA-sequence analysis, gene ontology (GO) analysis, WB, cell counting assay, invasion assays, and xenograft studies were conducted on these cells to investigate the biological roles of YAP1. IHC revealed that insulinoma-associated protein 1 was expressed in most cases (28 out of 32 cases), while only four cases expressed YAP1. The knockout of YAP1 in H69AR cells, a chemically induced SCLC-Y subtype, reduced cell proliferation and invasion capacity and restored drug sensitivity. Xenograft assays revealed that the knockout of YAP1 suppressed cell proliferation. Tumor tissues showed the expression of neuroendocrine markers and a low Ki-67 index. In SCLC, YAP1 plays an important role in biological functions, such as cell proliferation, EMT, drug sensitivity, and neuroendocrine differentiation.






Similar content being viewed by others
References
Bunn PA. Worldwide overview of the current status of lung cancer diagnosis and treatment. Arch Pathol Lab Med. 2012;136:1478–81.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification [Internet]. J. Thorac. Oncol. Elsevier Inc. 2015:1243–60 [cited 2021 Feb 25]. Available from: https://pubmed.ncbi.nlm.nih.gov/26291008/
Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–72.
Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol [Internet]. Mod Pathol 2012;25:18–30 [cited 2021 May 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/22214967/
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–97. https://doi.org/10.1038/s41568-019-0133-9.
Yamauchi T, Moroishi T. Hippo pathway in mammalian adaptive immune system. Cells. 2019;8:398.
Mo J, Park HW, Guan K. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 2014;15:642–56 [cited 2021 Jun 18]. Available from: /pmc/articles/PMC4197875/
Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015; 811–28 [cited 2021 May 30]. Available from: /pmc/articles/PMC4638384/
Moroishi T, Hansen CG, Guan K-L. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–9.
Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–85.
Yu M, Chen Y, Li X, Yang R, Zhang L, Huangfu L, et al. YAP1 contributes to NSCLC invasion and migration by promoting Slug transcription via the transcription co-factor TEAD. Cell Death Dis. 2018. https://doi.org/10.1038/s41419-018-0515-z.
Yoshida R, Nagata M, Nakayama H, Niimori-Kita K, Hassan W, Tanaka T, et al. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab Invest. 2013;93:1068–81 [cited 2021 Feb 25]. Available from: https://pubmed.ncbi.nlm.nih.gov/23938602/
Jiang L, Huang J, Higgs BW, Hu Z, Xiao Z, Yao X, et al. Genomic landscape survey identifies SRSF1 as a key oncodriver in small cell lung cancer. PLoS Genet 2016;12 [cited 2021 Feb 26]. Available from: https://pubmed.ncbi.nlm.nih.gov/27093186/
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013;8:2281–308 [cited 2021 Feb 25]. Available from: https://pubmed.ncbi.nlm.nih.gov/24157548/
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57 [cited 2021 Feb 25]. Available from: https://pubmed.ncbi.nlm.nih.gov/19131956/
Ito T, Kudoh S, Ichimura T, Fujino K, Hassan WAMA, Udaka N. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum Cel. 2017;30:1–10.
Gerlach JH. Multidrug resistance in a human small cell lung cancer cell line selected in Adriamycin. Cancer Res. 1987;47:2594–8.
Jho E. Dual role of YAP: oncoprotein and tumor suppressor. J Thorac Dis. 2018;10:S3895–8.
Dong X, Meng L, Liu P, Ji R, Su X, Xin Y, et al. YAP/TAZ: a promising target for squamous cell carcinoma treatment. Cancer Manag Res. 2019;11:6245–52.
Noguchi S, Saito A, Horie M, Mikami Y, Suzuki HI, Morishita Y, et al. An integrative analysis of the tumorigenic role of TAZ in human non-small cell lung cancer. Clin Cancer Res. 2014;20:4660–72.
Ito T, Matsubara D, Tanaka I, Makiya K, Tanei ZI, Kumagai Y, et al. Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci 2016;107:1527–38 [cited 2021 Feb 25]. Available from: https://pubmed.ncbi.nlm.nih.gov/27418196/
Horie M, Saito A, Ohshima M, Suzuki HI, Nagase T. YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Cancer Sci 2016;107:1755–66 [cited 2021 Feb 9]. Available from: /pmc/articles/PMC5198951/
Their JP. Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer. 2002;2:442–54.
Yu M, Chen Y, Li X, Yang R, Zhang L, Huangfu L, et al. YAP1 contributes to NSCLC invasion and migration by promoting Slug transcription via the transcription co-factor TEAD. Cell Death Dis 2018;9:1–13 [cited 2021 May 30]. Available from: https://www.nature.com/articles/s41419-018-0515-z
Hassan WA, Yoshida R, Kudoh S, Hasegawa K, Niimori-Kita K, Ito T. Notch1 controls cell invasion and metastasis in small cell lung carcinoma cell lines. Lung Cancer. 2014;86:304–10. https://doi.org/10.1016/j.lungcan.2014.10.007.
Hassan WA, Yoshida R, Kudoh S, Kameyama H, Hasegawa K, Niimori-Kita K, et al. Notch1 controls cell chemoresistance in small cell lung carcinoma cells. Thorac Cancer. 2016;7:123–8.
Acknowledgements
We thank Ms. Takako Maeda for her technical assistance, the staff of LILA for their technical support, and the Institute of Molecular Embryology and Genetics, Kumamoto University, for its help with RNA sequencing and Proteomics analyses. The present study was supported in part by the program of the Joint Usage/Research Center for Developmental Medicine, Institute of Molecular Embryology and Genetics, Kumamoto University, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20H03691), by a Grant from the Smoking Research Foundation, and by endowments from Dr. Yukoh Aihara of Aihara Allergy and Pediatric Clinic and from Prof. Kimitaka Itoh, Chubu University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All studies using human pathological samples followed the guidelines of the Ethics Committee of Kumamoto University (No. 342). All animal experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Kumamoto University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2022_669_MOESM1_ESM.tif
Supplementary Fig. S1 Molecules that correlate with YAP1. The RNA-seq dataset using tumor samples from 79 SCLC patients identified NOTCH1 and WNT7B as molecules that positively correlate with YAP1 in human SCLC tissues (TIF 1480 KB)
13577_2022_669_MOESM2_ESM.tif
Supplementary Fig. S2 YAP1-KO experiment in SBC5. The WB analysis showed that the knockout of the YAP1 gene in SBC5 cells increased SYP and decreased SLUG (TIF 2800 KB)
13577_2022_669_MOESM3_ESM.tif
Supplementary Fig. S3 Expression of YAP1 and NOTCH1. The WB analysis showed that the knockout of the Notch1 gene in H69AR cells reduced HES1. YAP1 was induced by the knockout of the Notch1 gene (TIF 2557 KB)
Rights and permissions
About this article
Cite this article
Saito, H., Tenjin, Y., Yamada, T. et al. The role of YAP1 in small cell lung cancer. Human Cell 35, 628–638 (2022). https://doi.org/10.1007/s13577-022-00669-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00669-6