Abstract
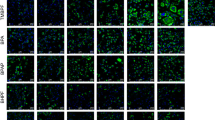
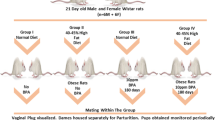
Environmental chemicals may contribute to the development of obesity and metabolic disorders such as diabetes. Bisphenol A (BPA) is one of the environmental chemicals that are widely used in daily life. This study was performed to investigate whether low dose BPA exposure can influence the occurrence of type II diabetes mellitus. Four weeks old Otsuka Long Evans Tokushima Fatty (OLETF) rats were randomly assigned to three groups of five animals and each group was given different concentrations of corn oil with BPA (0, 0.001, and 0.1 mg/kg/day). BPA 0.1 mg/kg/ day produced impairment of glucose tolerance, and induced higher insulin (p=0.028) and malondialdehyde levels (p=0.009) in serum than control group. Serum insulin levels in BPA 0.001 mg/kg/day treated group showed significantly higher than the control group (p=0.016). BPA tended to induce down-regulation of PPARγ mRNA and protein expression in white adipose tissue than control. In conclusion, low dose BPA exposed OLETF rats in adolescent period could accelerate the development of diabetes mellitus in younger adult period.
Similar content being viewed by others
References
Grun, F. & Blumberg, B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147, S50–55 (2006).
Newbold, R. R. et al. Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol. Carcino. 46, 783–796 (2007).
Baillie-Hamilton, P. F. Chemical toxins: a hypothesis to explain the global obesity epidemic. J. Altern. Complement. Med. 8, 185–192 (2002).
Dahlman-Wright, K. et al. International union of pharmacology. LXIV. Estrogen receptors. Pharmacol. Rev. 58, 773–781 (2006).
Shelby, M. D. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Ntp. Cerhr. Mon., v, vii–ix, 1-64 passim (2008).
Fernandez, M. F. et al. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 24, 259–264 (2007).
Dekant, W. & Volkel, W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 228, 114–134 (2008).
Vandenberg, L. N. et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 118, 1055–1070 (2010).
Carwile, J. L. & Michels, K. B. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ. Res. 111, 825–830 (2011).
Wang, T. et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 97, E223–227 (2012).
Masuno, H., Iwanami, J., Kidani, T., Sakayama, K. & Honda, K. Bisphenol A accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol. Sci. 84, 319–327 (2005).
Somm, E. et al. Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ. Health Perspect. 117, 1549–1555 (2009).
Miyawaki, J., Sakayama, K., Kato, H., Yamamoto, H. & Masuno, H. Perinatal and postnatal exposure to bisphenol A increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 14, 245–252 (2007).
National Toxicology Program (NTP). http://ntp.niehs.nih.gov/ntp/htdocs/liason/LowDosePeerFinalRpt.pdf (2001).
Honma, S. et al. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod. Toxicol. 16, 117–122 (2002).
Alonso-Magdalena, P. et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect. 118, 1243–1250 (2010).
Roy, J. R., Chakraborty, S. & Chakraborty, T. R. Estrogen-like endocrine disrupting chemicals affecting puberty in humans-a review. Med. Sci. Monit. 15, RA137–145 (2009).
Lee, C. H., Olson, P. & Evans, R. M. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144, 2201–2207 (2003).
Auwerx, J. PPARgamma, the ultimate thrifty gene. Diabetologia 42, 1033–1049 (1999).
Janesick, A. & Blumberg, B. Minireview: PPARgamma as the target of obesogens. J. Steroid Biochem. Mol. Biol. 127, 4–8 (2011).
Kawano, K. et al. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41, 1422–1428 (1992).
Moran, T. H. & Bi, S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans. R. Soc. Lond. B Biol. Sci. 361, 1211–1218 (2006).
Nakaya, Y. et al. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am. J. Clin. Nutr. 71, 54–58 (2000).
Choi, K. C. et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem. Biophys. Res. Commun. 336, 747–753 (2005).
Nagai, N., Murao, T., Okamoto, N. & Ito, Y. Disulfiram reduces elevated blood glucose levels in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of type 2 diabetes. J. Oleo Sci. 58, 485–490 (2009).
Howdeshell, K. L., Hotchkiss, A. K., Thayer, K. A., Vandenbergh, J. G. & vom Saal, F. S. Exposure to bisphenol A advances puberty. Nature 401, 763–764 (1999).
Masuno, H. et al. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid Res. 43, 676–684 (2002).
Nagel, S. C. et al. Relative binding affinity-serum modi-fied access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ. Health Perspect. 105, 70–76 (1997).
Kabuto, H., Amakawa, M. & Shishibori, T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 74, 2931–2940 (2004).
Alonso-Magdalena, P., Morimoto, S., Ripoll, C., Fuentes, E. & Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 114, 106–112 (2006).
Wei, J. et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 152, 3049–3061 (2011).
Ding, S. et al. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J. Endocrinol. 221, 167–179 (2014).
Dodge, J. A. et al. Environmental estrogens: effects on cholesterol lowering and bone in the ovariectomized rat. J. Steroid Biochem. Mol. Biol. 59, 155–161 (1996).
Seidlova-Wuttke, D., Jarry, H., Christoffel, J., Rimoldi, G. & Wuttke, W. Effects of bisphenol-A(BPA), dibutylphtalate (DBP), benzophenone-2 (BP2), procymidone (Proc), and linurone (Lin) on fat tissue, a variety of hormones and metabolic parameters: a 3 months comparison with effects of estradiol (E2) in ovariectomized (ovx) rats. Toxicology 213, 13–24 (2005).
Ben-Jonathan, N., Hugo, E. R. & Brandebourg, T. D. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol. Cell. Endocrinol. 304, 49–54 (2009).
Rosen, P. et al. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCOMCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev. 17, 189–212 (2001).
Bindhumol, V., Chitra, K. C. & Mathur, P. P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188, 117–124 (2003).
Grun, F. et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 20, 2141–2155 (2006).
Chamorro-Garcia, R. et al. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferatoractivated receptor gamma-independent mechanism. Environ. Health Perspect. 120, 984–989 (2012).
Kim, S. Y. The effects of low-dose exposure of di(2-ethylhexyl)phthalate, bisphenol A, bisphenol A diglycidyl ether on glucose metabolism and thyroid hormone in sprague-dawley rats and Otsuka Long-Everns Tokushima Fatty rats, Master’s degree thesis, Chung-Ang University (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, Yj., Kim, Sy., Hong, Yp. et al. Environmentally relevant levels of Bisphenol A may accelerate the development of type II diabetes mellitus in adolescent Otsuka Long Evans Tokushima Fatty rats. Toxicol. Environ. Health Sci. 6, 41–47 (2014). https://doi.org/10.1007/s13530-014-0186-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-014-0186-9