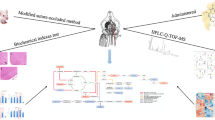
Abstract
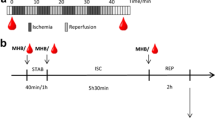
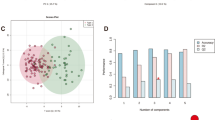
Cardiac arrest is one of the leading causes of death among adults in older age. Understanding mechanisms how organism responds to ischemia at global level is essential for the prevention and ischemic patient’s treatment. In this study, we used a global cerebral ischemia induced by four-vessel occlusion as an established animal model for ischemic stroke to investigate metabolic changes after 24 h reperfusion, when transitions occur due to the onset of delayed neuronal death. We also focused on the endogenous phenomenon known as ischemic tolerance by the pre-ischemic treatment. The experiments were carried out on blood plasma samples as easily available and metabolically reflecting the overall changes in injured organism. Our results imply that disturbed glycolysis pathway, as a consequence of ischemic injury, leads to the increased level of ketone bodies (acetone, acetoacetate and β-hydroxybutyrate) along with increased utilization of triacylglycerols in plasma of ischemic and ischemically preconditioned rats. Complementary to, a decreased level of glycolytic intermediates (lactate, pyruvate, acetate) with increased level of glucose was found in ischemic and preconditioned animals. The protective effect of ischemic preconditioning on metabolome recovery was demonstrated by significantly increased level of creatine compared to ischemic, non-preconditioned rats. We also document that acetoacetate, pyruvate, lactate, and leucine have the best discriminatory power between ischemic and control plasma. Conclusively, our results provide evidence that NMR spectra analysis can identify specific group of metabolites present in plasma with the capability for discrimination between individual groups of animals. In addition, an excellent feasibility for the statistical discrimination among ischemic, preconditioned, and control rats can be applied regardless of native or deproteinated plasma and also regardless of noesy or cpmg NMR acquisition.






Similar content being viewed by others
References
Fluri F, Schuhmann MK, Kleinschnitz C (2015) Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther 9:3445–3454. https://doi.org/10.2147/DDDT.S56071.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7.
Lehotsky J, Burda J, Danielisova V, Gottlieb M, Kaplan P, Saniova B (2009) Ischemic tolerance: the mechanism of neuroprotective strategy. Anat Rec 292:2002–2012. https://doi.org/10.1002/ar.20970
Dirnagl U, Becker K, Mesel A (2009) Preconditioning and tolerance against cerebral ischemia, from experimental strategies to clinical use. Lancet Neurol 8(4):398–412. https://doi.org/10.1016/S1474-4422(09)70054-7
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Barallobre-Barreiro J, Chung YL, Mayr M (2013) Proteomics and metabolomics for mechanistic insights and biomarker discovery in cardiovascular disease. Rev Esp Cardiol (Engl Ed) 66(8):657–661. https://doi.org/10.1016/j.rec.2013.04.009
Griffin JL, Atherton H, Shockor J, Atzori L (2011) Metabolomics as a tool for cardiac research. Nat Rev Cardiol 8(11):630–543. https://doi.org/10.1038/nrcardio.2011.138
Yin P, Lehmann R, Xu G (2015) Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal Bioanal Chem 407(17):4879–4892. https://doi.org/10.1007/s00216-015-8565-x
Liu M, Tang L, Liu X, Fang J, Zhan H, Wu H, Yang H (2016) An evidence-based review of related metabolites and metabolic network research on cerebral ischemia. Oxid Med Cell Longev (ID 9162074). https://doi.org/10.1155/2016/9162074
White H, Venkatesh B (2011) Clinical review: ketones and brain injury. Crit Care 15(2):219. https://doi.org/10.1186/cc10020
Jouret F, Leenders J, Poma L, Defraigne JO, Krzesinski JM (2016) Nuclear magnetic resonance metabolomic profiling of mouse kidney, urine and serum following renal ischemia/reperfusion injury. PLoS One 2016:e0163021. https://doi.org/10.1371/journal.pone.0163021
Kovalska M, Kovalska L, Mikuskova K, Adamkov M, Tatarkova Z, Lehotsky J (2014) p-ERK involvement in the neuroprotection exerted by ischemic preconditioning in rat hippocampus subjected to four vessel occlusion. J Physiol Pharmacol 65(5):767–776
Beckonert O, Keun HC, Ebbels TMD, Bundy J, Holmes E, Lindon JC, Nicholson JK (2007) Metabolic profiling, metabolomics and metabonomic. Nat Prootoc 2(11):2692–2703
Kohl SM, Klein MS, Hochrein J, Oefner PJ, Spang R, Gronwald W (2012) State-of-the art data normalization methods improve nmr-based metabolomic analysis. Metabolomics 8(Suppl 1):146–160
Xia J, Sinelnikov I, Han B, Wishart DS (2016) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 43(W1):W251–W257. https://doi.org/10.1093/nar/gkv380
Breiman L (2001) Random forests. Mach Learn 45:5–32. https://doi.org/10.1023/A:1010933404324.
Development Core Team R (2015) R: a language and environment for statistical computing. R foundation for statistical computing. the R Foundation for Statistical Computing, Vienna, Austria ISBN: 3-900051-07-0
Kuhn M. (2016) Classification and regression training, R package version. https://CRAN.R-project.org/package=caret
Kuhn M, Johnson K (2013) Applied predictive modeling. Springer
Fleiss JL, Levin B, Paik MC (2013) Statistical methods for rates and proportions. John Wiley & Sons
Wacker WEC, Ulmer DD, Vallee BLN (1956) Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med 255:449–456
Karmen A, Wroblewski F, LaDue JS (1954) Transaminase activity in human blood. J Cli Invest 34(1):126–133. https://doi.org/10.1172/JCI103055
Hendriks MM, van Eeuwijk FA, Jellema RH, Westerhuis JA, Reijmers TH, Hoefsloot HC, Smilde AK (2011) Data-processing strategies for metabolomics studies. Trends Anal Chem 30:1658–1698
Shasi AL, Amin A, Adeghate E (2006) Effect of vitamin C on liver and kidney functions in normal and diabetic rats. Ann N Y Acad Sci 1084:371–390. https://doi.org/10.1196/annals.1372.031
Guyon I, Elisee A (2013) An introduction to variable and feature selection. J Machine Learning Res 3:1157–1182
Bartel J, Krumisiek J, Theis FJ (2013) Statistical methods for the data analysis of high-throughput metabolomics data. CSBJ 4(5):1–9. https://doi.org/10.5936/csbj.201301009
Wold S, Ebsensen K, Geladi P (1987) Principal component analysis. Chemometr Intell Lab Syst 2:37–52. https://doi.org/10.1016/0169-7439(87)80084-9
Worley B, Powers R (2013) Multivariate analysis in metabolomics. Curr Metabolomics 1(1):92–107. https://doi.org/10.2174/2213235X11301010092.
Chang LH, Shimizu H, Abika H, Swason RA, Faden AI, James TL, Weinstein PR (1992) Effect of dichloroacetate on recovery of brain lactate, phosphorus energy metabolites, and glutamate during reperfusion after complete cerebral ischemia in rats. J Cereb Blood Flow Metab 12(6):1030–1038
Zheng Y, Wang XM (2017) Measurement of lactate content and amide proton tranfer values in the basala ganglia of neonatal piglet hypoxic-ischemic brain injury model using MRI. AJNR Am J Neuroradiol 38(4):827–824. https://doi.org/10.3174/ajnr.A5066
Rehncrona S, Rosen I, Siesjo BK (1981) Brain lactic acidosis and ischemic cell damage: 1. Biochemistry and neurophysiology. J Cereb Blood Flow Metab 1(3):297–311
Hall G, Stromstand M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB (2009) Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29(6):1121–1129. https://doi.org/10.1038/jcbfm.2009.35
Chatham JC (2002) Lactate—the forgotten fuel. J Physiol 542(Pt 2):333
Ginsberg MD, Globus MYT, Dietrich D, Busto R (1993) Temperature modulation of ischemic brain injury—a synthesis of recent advances. Prog Brain Res 96:13–22
Christensen H, Boysen G (2002) Blood glucose increases early after stroke onset: a study on serial measurements of blood glucose in acute stroke. Eur J Nerol 9(3):297–301
Suzuki M, Suzuki M, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A (2001) Effect of beta-hydroxybutyrate, a cerebral function improving agent on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn J Pharmacol 87(2):134–150
Drgova A, Likavčanová A, Dobrota D (2004) Changes of phospholipid composition and superoxide dismutase activity during global brain ischemia and reperfusion in rats. Gen Physiol Biophycs 23(3):337–346
Prins ML (2008) Cerebral metabolic adaptation and ketone metabolism after brain injury. J Cereb Blood Flow Metab 28(1):1–16. https://doi.org/10.1038/sj.jcbfm.9600543
Springer J, Schust S, Peske K, Tschirner A, Rex A, Engel O, Scherbakov N, Meisel A, von Haehling S, Boschmann M, Anker SD, Dirnagl U, Doehner W (2014) Catabolic signaling and muscle wasting after acute ischemic stroke in mice. Stroke 45(12):3675–3683. https://doi.org/10.1161/STROKEAHA.114.006258
Koch K, Berressem D, Konietzka J, Thinnes A, Eckert GP, Klein J (2017) Hepatic ketogenesis induced by cerebral artery occlusion in mice. J Am Heart Assoc 6(4):e005556. https://doi.org/10.1161/JAHA.117.005556
Brody T (1999) Nutritional biochemistry, 2nd edn. Academic Press, Berkeley, California
Datta D, Verma P, Banerjee A, Kar S, Sengupta T, Sengupta N, Kumar SS, Khan EM (2016) Lysine as a potential low molecular weight angiogen: its clinical, experimental and in-silico validation—a brief study. bioRxiv doi: https://doi.org/10.1101/080176
Danne O, Lueders C, Storm C, Frei U, Mockel M (2007) Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta 383(1–2):103–109
Jin X, Wang R, Wang H, Long C, Wang H (2015) Brain protection against ischemic stroke using choline as a new molecular bypass treatment. Acta Pharmacol Sin 36(12):1416–1425. https://doi.org/10.1038/aps.2015.104.
Scremin OU, Jenden DJ (1991) Time-dependent changes in cerebral choline and acetylcholine induced by transient global ischemia in rats. Stroke 22(6):643–647
Barcelos RP, Stefanello ST, Mauriz JL, Gonzalez-Gallego J, Soares FAA (2016) Creatine and the liver: metabolism and possible interactions. Mini Rev Med Chem 16(1):12–18
Kitzenberg D, Colgan SP, Glover LE (2016) Creatine kinase in ischemic and inflammatory disorders. Clin Transl Med 5(1):31
Lensman M, Korzhevskii DE, Mourovets VO, Kostkin VB, Izvarnina N, Perasso L, Gandolfo C, Otellin VA, Polenov SA, Balestrino M (2006) Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res 1114(1):187–194
Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P, Flint Beal M, Brown RH, Kristal BS, Ferrante RJ, Friedlander RM (2004) Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci 24(26):59009–59012
Wyss M, Kaddurah-Daouk R Creatine and Creatinine Metabolism (2000) Physiol Rev 80(3):1107 213
Gavino V, Somma J, Philbert L, David F, Garneaut M, Belair J, Brunengraber H (1987) Production of acetone and conversion of acetone to acetate in the perfused rat liver. J Biol Chem 262(14):6735–6740
Kosugil K, Scofield RF, Chandramouli V, Kumaran K, Schumann WC, Landau BR (1986) Pathways of acetone’s metabolism in the rat. JBC 261(9):3952–3957
Acknowledgments
This work was supported by the project “Biomedical Center Martin” ITMS code 26220220187, the project is co-financed from EU sources, Slovak Research and Development Agency (APVV) grant number APVV 15/0107, Slovak Scientific Grant Agency grant number VEGA 128/16, and “Identification of Novel Markers in Diagnostic panel of Neurological Diseases” code: 26220220114. This work was also supported by the project “ Center of Translational Medicine,” ITMS: 26220220021, co-funded from EU sources and European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary table
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Baranovicova, E., Grendar, M., Kalenska, D. et al. NMR metabolomic study of blood plasma in ischemic and ischemically preconditioned rats: an increased level of ketone bodies and decreased content of glycolytic products 24 h after global cerebral ischemia. J Physiol Biochem 74, 417–429 (2018). https://doi.org/10.1007/s13105-018-0632-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0632-2