Abstract
In these experiments, we tested the hypothesis that inhibition of the estrogen receptor (ER) with Tamoxifen and activation of PPARγ with fish oil (FO) rich in omega-3 (n-3; known PPAR agonists) inhibit the development of hormone-independent breast cancer in view of the known crosstalk between the ER and PPARγ pathways. We selected the polyoma middle T transgenic mouse model, since in this system the development of ER− tumors is preceded by ER positive preneoplastic lesions. Tamoxifen admixed with a 20% corn oil (CO) modified AIN-76A diet delayed mammary carcinogenesis and inhibited tumor multiplicity, volume, and weight in a dose-dependent (1, 10, and 100 ppm) fashion. Administration of increasing concentrations of FO in the diet (5%, 10%, and 17%) did not affect any of the tumor parameters. Combined administration of different doses of Tamoxifen and FO delayed carcinogenesis and suppressed tumor multiplicity and volume to the same extent as Tamoxifen alone. Mice fed 10% FO exhibited the expected increase in n-3/n-6 ratio in plasma and tumor based on diet analysis. Further increase in the n-3/n-6 ratio was not observed in mice fed the 17% FO diet. FO reduced tissue levels of arachidonic acid and its metabolite PGF-2α. Our results support the role of ER expression by preneoplastic lesions in the development of hormone-independent tumors and consequently the importance of including ER targeting in combination with mechanistically based novel chemopreventive agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estrogen receptor expression has been shown to be upregulated in premalignant human mammary lesions [1]. Such event may accelerate the estrogen-induced proliferation of initiated ER positive breast epithelial cells. These cells may then accumulate additional mutations as a result of their increased growth rate eventually leading to the development of ER positive breast cancer. Targeting ER with antiestrogens such as Tamoxifen and Raloxifene has indeed been shown to be effective in preventing ER positive tumors [2, 3]. These agents, however, are ineffective in inhibiting the development of hormone-independent breast cancer.
We postulate that upregulation of ER may also contribute to the development of hormone-independent tumors through cross-activation of various growth factor signaling pathways which can lead to hormone independence by bypassing the need for estrogen stimulation. This mechanism of hormone resistance is well documented in breast cancer models where the concomitant targeting of ER and interacting oncogenic pathways has been shown to have a superior antitumor effect, preventing or delaying the development of antiestrogen resistance [4, 5]. On the basis of these experimental data, this combination approach is being tested in therapeutic clinical trials [5].
Our laboratories have been interested in testing the hypothesis that similar mechanisms may be operating during mammary carcinogenesis and that such a combined approach may improve the chemopreventive effects of antiestrogens, possibly to include prevention of hormone-independent tumors. We have recently reported that a combination of a fish oil (FO)-rich diet and Tamoxifen inhibited N-methyl-N-nitrosourea (MNU)-induced rat mammary carcinogenesis to a greater extent than the individual interventions [6]. The rationale for testing this combined strategy is based upon the documented crosstalk between the ER and PPARγ receptors for which omega-3 fatty acids (n-3 PUFA), present in FO, are natural ligands [7, 8]. n-3 PUFA may be protective against some common cancers, including breast cancer, although the evidence supporting their protective effect remains controversial [9]. In addition, diet enriched with FO may have added health benefits such as a reduction in cardiovascular risk [10]. Therefore, n-3 PUFA is an attractive class of compounds to be used in combination with antiestrogens for breast cancer prevention.
The present experiments were designed to test the chemopreventive efficacy of the individual and combined administration of Tamoxifen and n-3 PUFA on mammary carcinogenesis in Polyoma Middle T transgenic mice. Based on their potential mechanism of interaction, we evaluated the effects of these interventions on the expression of select genes in the estrogen and PPAR pathways. We selected the polyoma middle T transgenic model since it has been shown to exhibit progressive stages of breast cancer development similar to those observed in humans [11]. Furthermore, and most importantly, in this experimental system functional estrogen receptors have been found to be markedly upregulated in the preneoplastic lesions, whereas their expression is lost in most invasive adenocarcinomas [11]. Therefore, this model is particularly suitable for examining the value of targeting the ER in the preneoplastic lesions jointly with other molecules that have complementary mechanisms of action in order to inhibit the development of hormone-independent tumors.
Materials and Methods
Mice
FVB mice expressing the polyoma middle T (PyMT) antigen under control of the mouse mammary tumor virus long terminal repeat established by Dr. WJ Muller et al. [12] were obtained from NCI-Frederick. Female carriers develop palpable mammary adenocarcinomas by 5 weeks of age. The tumors are multifocal, highly fibrotic, and involve the entire mammary fat pad. Since female PyMT mice fail to lactate, this strain is maintained by breeding hemizygous males with FVB/N wild-type females. The presence of the transgene was routinely checked by PCR on tail DNA following the protocol from NCI-Frederick. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine.
Diets
Modified AIN-76A diets were developed by Test Diet. All dietary ingredients were obtained from Dyets except for Virginia Prime Gold Fish Oil (Omega Protein). Over the course of our experiments, two different batches of FO were used. The diets were prepared monthly in our laboratory, flushed with nitrogen, and stored at 4°C until use. In the Tamoxifen-treated groups, Tamoxifen was dissolved in ethanol, admixed with the oil (2% v/w), and added to the diet. All the diets were isocaloric (4.78 kcal/g). The percent of energy from proteins, carbohydrates, and fat was 20%, 42%, and 38%, respectively. The composition of the diets as well as the percent of energy derived from saturated, monounsaturated, and polyunsaturated fatty acids are shown in Supplementary Table 1. The calculated n-3/n-6 ratio was 0.005 in the 20% CO diet, 0.2 in the 5% FO + 15% CO diet, 0.56 in the 10% FO + 10% CO diet, and 2.3 in the 17% FO + 3% CO diet. In addition, we performed FA analysis of select diet samples used in our experiments. Specifically, since we used two different FO batches, we wanted to verify that the composition of our FO-rich diets was not affected by this potential variable (Supplementary Table 2).
Experimental Design
Tamoxifen Dose-Finding Studies in Wild-Type Mice
These experiments were performed to define the antiestrogens effects and safety profile of the oral administration of Tamoxifen to wild-type mice in order to select appropriate doses of the drug for our chemoprevention studies. After weaning at 3 weeks, separate groups of mice (n = 10/group) were placed on a 20% CO diet containing increasing concentrations of Tamoxifen (1, 100, 200, and 500 ppm) or no drug until they were sacrificed by CO2 asphyxiation at week 14 of age. Body weight was monitored weekly throughout the duration of the experiment. At the time of sacrifice, blood was obtained by cardiac puncture and serum was separated for measurement of liver enzymes. Uteri were removed and their weights recorded as biomarkers of the antiestrogenic action of Tamoxifen. Livers were also collected, weighed and processed for routine histology to assess possible Tamoxifen toxicity.
Tamoxifen and Fish Oil Chemoprevention Dose–Response Studies
In these experiments, we tested the dose dependency of the chemopreventive actions of Tamoxifen and FO administered individually to polyoma middle T transgenic mice. After weaning at 3 weeks of age, groups of transgenic mice (n = 13–14/group) were randomly assigned to one of the following experimental interventions: (1) 20% CO, (2) 20% CO + Tamoxifen 1 ppm, (3) 20% CO + Tamoxifen 10 ppm, (4) 20% CO + Tamoxifen 100 ppm, (5) 5% FO + 15% CO, (6) 10% FO + 10% CO, and (7) 17% FO + 3% CO. Body weights and tumor development were monitored weekly until the mice were sacrificed by CO2 asphyxiation at week 11 of age. At termination, tumors were removed and their volume was determined using the formula \( V = \frac{{{\text{width}}2 \times {\text{length}} \times \pi }}{6} \). In addition, the tumors were weighed and processed for routine histology.
Combination Tamoxifen and Fish Oil Chemoprevention Studies
In the first experiment, groups of 3-week-old transgenic mice (n = 14–15/group) were randomly assigned to one of the following experimental interventions: (1) 20% CO, (2) 20% CO + Tamoxifen 100 ppm, (3) 10% FO + 10% CO, and (4) 10% FO + 10% CO + Tamoxifen 100 ppm. At week 11 of age, half of the mice in each group was sacrificed by CO2 asphyxiation, while the other half was followed for an additional 2 weeks. In addition, Tamoxifen at 100 ppm was added to the diet of the mice in groups 1 and 3. In the second experiment, groups of 3-week-old transgenic mice (n = 12/group) were randomized to one of the following interventions: (1) 20% CO, (2) 20% CO + Tamoxifen 1 ppm, (3) 17% FO + 3% CO, and (4) 17% FO + 3%CO + Tamoxifen 1 ppm. At week 11 of age, all mice were sacrificed by CO2 asphyxiation. In both experiments, body weights were recorded weekly and tumor development was also monitored weekly by palpation. At termination, tumors were removed, their volumes were recorded, and the tumors were processed for routine histologic analysis. In addition, aliquots of frozen tumors from randomly selected mice fed 20% CO, 10% FO + 10% CO, and 17% FO + 3% CO were obtained for determination of tissue fatty acid composition, measurement of PGF-2α, and analysis of expression of select PPAR-related genes. Furthermore, analysis of expression of select estrogen related genes was performed in randomly selected tumors from rats fed different diets in the presence and in the absence of Tamoxifen 100 ppm. Finally, plasma fatty acid analysis was performed in groups of mice fed the three different diets.
Fatty Acid Analysis in the Diets, Plasma, and Tumors
Identification and quantification of the total plasma and tumor fatty acids were determined by gas chromatography equipped with flame ionization detection using authentic standards as described previously [6, 13]. The identification of fatty acid methyl esters was accomplished by comparison with the relative retention time of standards. Quantification was based on internal standard calculations as compared to calibration analyses with authentic standards.
Measurement of Tumor PGF-2α
PGF2α was determined in tissue supernates taken from a 5% tissue homogenate prepared at −20°C. The immunoassay used is a high sensitive competitive microtiter plate enzyme immunoassay with reagents obtained from Enzo Life Sciences (Plymouth Meeting, PA). The PGF2α used is for the quantitative determination of Prostaglandin F2α in culture supernates, tissue homogenates, saliva, urine, serum, and plasma. Standards and samples are added to wells coated with a DxS IgG antibody. A yellow solution of sheep polyclonal antibody to PGF2α is then added, followed by a blue solution of PGF2α conjugated to alkaline phosphatase. During a simultaneous overnight incubation at 4°C, the antibody to PGF2α binds, in a competitive manner with the PGF2α in the sample or conjugate. The plate is washed, leaving only bound PGF2α. A substrate solution of para nitro phenol phosphate solution is added. The color reaction due to the alkaline phosphatase in the conjugate is read at 405 nm and the amount of signal is indirectly proportional to the amount of PGF2α in the sample. Results were determined from a log/logit transformation of the standard curve and expressed as pg per milligram of tissue protein. The assay showed minimal cross reactivity with other potentially interfering eicosanoids such as PGE2, 6-keto PGF1a, or thromboxane B2. Analytical sensitivity of the assay is 1 pg/ml with inter-assay imprecision averaging 8% at a concentration of 35 pg/ml.
Gene Expression Analysis
Quantitative real-time PCR was performed essentially as previously described [14]. Briefly, total RNA was reverse transcribed using the ABI High Capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Standard curves were made using serial dilutions from pooled cDNA samples. Real-time PCR was performed using the SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s protocol and run on the ABI Prism 7300 Sequence Detection System. Primer sequences are given in Supplementary Table 3. Quantification was performed for each gene and normalized to a housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase).
Statistical Methods
Regression methods were used to analyze the effects of Tamoxifen and different diets on the outcome variables shown in the figures and tables. Mixed effects regression was used to model longitudinal data such as repeated measurements of body weight and number of palpable lesions. Analysis of variance (ANOVA) was used to evaluate the effects of different diets on plasma and tumor fatty acids profile. ANOVA was performed on SAS 9.2. For quantitation of gene expression by RT-PCR, normalized expression values were examined by ANOVA and Tukey’s post hoc tests as necessary. Statistical analysis was performed in the JMP 7 software (SAS Institute, Cary, NC). Differences were considered statistically significant with a p value less than 0.05.
Results
Tamoxifen Dose-Finding Studies in Wild-Type Mice
In order to determine the appropriate doses of Tamoxifen for our chemoprevention experiments, we initially performed a dose–response study in wild-type mice to define the antiestrogenic activity and safety profile of oral Tamoxifen administration.
As can be seen in Fig. 1a, uterine weights, selected as biomarkers of an estrogen effect, were significantly reduced (p < 0.0001) at each of the Tamoxifen doses tested. The antiestrogenic effect of Tamoxifen was already apparent at the 1 ppm dose and reached its maximum at 100 ppm. All doses of Tamoxifen similarly reduced body weight gain compared to untreated mice (Fig. 1b; p < 0.0001), an effect previously observed by us [6] and other investigators [15, 16] and shown not to reflect toxicity from the drug. The absence of Tamoxifen toxicity is further supported by the lack of change in liver weight and liver enzymes (Fig. 1c) in treated mice compared to control. Tamoxifen administration did not alter liver histology, except for inducing mild changes of biliary hyperplasia which have been reported in rats receiving Tamoxifen [17] an effect of limited biological significance.
Effect of increasing doses of Tamoxifen on uterine weights (a), body weights (b), and liver weights and enzymes (c) in wild-type mice. Groups of 3-week-old wild-type mice (n = 10/group) were placed on a 20% CO diet containing the indicated doses of Tamoxifen or no drug until they were sacrificed at week 14 of age (see “Materials and Methods” section for details). Data are expressed as means ± SE
Dose–Response Chemopreventive Studies with Tamoxifen and Fish Oil in Polyoma Middle T Transgenic Mice
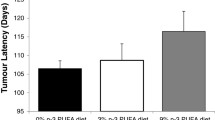
Tamoxifen delayed in a dose-dependent fashion the development of mammary carcinomas (Fig. 2a). Although tumor incidence at termination was 100% in all experimental groups, Tamoxifen treatment at doses of 10 and 100 ppm significantly (p < 0.0001) reduced the incidence of palpable lesions (Fig. 2a) and the number of palpable lesions per mouse over time (Fig. 2b) compared to untreated mice or mice receiving 1 ppm Tamoxifen, the number of histologically confirmed adenocarcinomas at sacrifice (Table 1; p < 0.0002) as well as tumor volume and tumor weight/mouse (Table 1; p < 0.0001 for both). FO administration, on the other hand, did not affect any of these parameters (Fig. 2c, d and Table 1) at the doses tested.
Effect of increasing concentrations of Tamoxifen (TAM) (panels a and b) and fish oil (panels c and d) on tumor development (panels a and c) and multiplicity (panels b and d). Groups of 3-week-old transgenic mice (n = 13–14/group) were randomized to increasing concentrations of Tamoxifen or fish oil as indicated. Tumor development was monitored weekly by palpation
Chemoprevention Studies with Individual and Combined Administration of Tamoxifen and Fish Oil in Polyoma Middle T Transgenic Mice
In the first experiment, we used a dose of Tamoxifen of 100 ppm, which was found to have a maximum antiestrogenic effect (Fig. 1a) and a dose of FO of 10%, reported to have a significant chemopreventive effect in the MNU rat mammary tumor model [18, 19]. As can be seen in Fig. 3, Tamoxifen significantly delayed the development of palpable lesions (Fig. 3a) and their multiplicity over time (Fig. 3b; p < 0.0001), whereas FO had no effect on either parameter whether administered alone or in combination with Tamoxifen (Fig. 3). Of interest, when Tamoxifen was given to mice with established tumors, it did not inhibit tumor growth (Fig. 4). In the aggregate, the effects of Tamoxifen are consistent with the known overexpession of ER in the preneoplastic proliferative lesions and their absence in the vast majority of invasive cancers [11].
Effect of Tamoxifen (TAM) 100 ppm and 10% fish oil (FO) individually and in combination on tumor development (a) and multiplicity (b). Groups of 3-week-old transgenic mice were randomly assigned to the indicated experimental interventions and monitored weekly for the development of palpable tumors. At week 11, approximately half of the mice in each group were sacrificed and assessed for the presence of tumors at autopsy (data shown in Table 2) while the remaining mice were followed for an additional 2 weeks to evaluate the effects of Tamoxifen on the growth of established tumors (data shown in Fig. 4). See text for further experimental details
Effect of Tamoxifen (TAM) 100 ppm on the growth of established tumors. Groups of 3-week-old transgenic mice were randomly assigned to the indicated experimental interventions. At week 11 of age, Tamoxifen 100 ppm was added to the diet of mice fed 20% CO or 10% FO. The mice were followed for an additional 2 weeks prior to sacrifice. See “Materials and Methods” section and the legend to Fig. 3 for further experimental details
Table 2 reports the effects of our interventions on tumor multiplicity and volume documented at termination. As can be seen, Tamoxifen at 100 ppm reduced both the number of tumors and the tumor volume per mouse (p < 0.0001 for both), whereas FO at 10% had no effect on either parameter when given alone or in combination with Tamoxifen.
In the second experiment, we used a dose of Tamoxifen of 1 ppm that has suboptimal antiestrogenic and antitumor effect and a dose of FO of 17%, which we found potentiated the chemopreventive action of Tamoxifen in a prepubertal model of MNU-induced rat mammary carcinogenesis [6].
As expected, Tamoxifen at 1 ppm did not delay tumorigenesis (Fig. 5a), although it reduced the number of palpable lesions developing over time (Fig. 5b; p < 0.03). Tamoxifen at 1 ppm did not affect tumor multiplicity but reduced tumor volume at termination (p < 0.02; Table 3). FO at 17% had no effect on any of these parameters by itself or in combination with Tamoxifen (Fig. 5 and Table 3).
Effect of FO-Rich Diets on Plasma and Tumor FA Concentrations
To analyze the impact of dietary FO on the fatty acid profiles in mammary tissue and plasma, we measured the levels of individual FA expressed in both absolute concentrations (per ml of plasma or per g of tissue) and their relative concentrations (percent of total FA) for mice in each of the three diet groups (20% CO, 10% FO + 10% CO, and 17% FO + 3% CO [Supplementary Table 4]). As can be seen in Supplementary Table 4, mice fed the 10% FO diet exhibited the expected changes in plasma FA composition. This consisted of an increase in n-3 levels and a decrease in n-6 levels resulting in a nearly sevenfold increase in the n-3/n-6 ratio. Remarkably, the plasma n-3/n-6 ratio (0.34) was virtually identical to the ratio measured in the 10% FO diet samples (0.39 and 0.32; Supplementary Table 2). Overall, similar changes were observed in the mammary tumors, although the variability was larger and precluded some of the changes from achieving statistical significance (Supplementary Table 4).
Surprisingly, raising the amount of FO in the diet to 17% did not increase these effects but instead tended to reduce them. This was particularly evident in the tumors, where the n-3/n-6 ratio (0.08) was significantly lower than that measured in the tumors of the mice fed 10% FO (0.29) and was essentially identical to that of the tumors of the mice fed the 20% CO control diet (0.05; Supplementary Table 4). These surprising results could not be explained by major differences in diet composition resulting from the use of different batches of FO or other unknown variables occurring in diet preparation. As can be seen in Supplementary Table 2, the composition of the diets used for feeding the 10% FO group was similar to that used for feeding the 17% FO group (Supplementary Table 2).
Effect of FO-Rich Diets on Tumor PGF-2α Level
To determine the downstream effects of FO on fatty acid metabolism, we analyzed the tissue levels of PGF-2α, an arachidonic acid derived eicosanoid. As can be seen in Fig. 6, FO caused a dose-dependent decrease in the PGF-2α level, consistent with the observed dose-dependent reduction in tissue arachidonic acid (Supplementary Table 4).
Dietary effects on tumor levels of PGF-2α (n = 10/group). Bars represent means ± SE. See “Materials and Methods” section for experimental details. Twenty percent CO group was significantly different from 10% FO (p = 0.001) and 17% FO (p < 0.0001) groups
Effect of FO and TAM on Gene Expression
As can be seen in Fig. 7, Tamoxifen did not significantly affect mRNA levels of certain estrogen related genes (AREG, KRT19, GAS6, FGB, PGR) in the tumors of mice fed either a CO- or a FO-rich diet. This is not surprising since by the time the tumors were harvested, they had become resistant to Tamoxifen (Fig. 4). In agreement with the lack of effect of FO on carcinogenesis in our experimental system, neither of the two FO-rich diets (10% FO and 17% FO) modified the tumor expression of several PPAR-related genes (PPARα, PPARβ/δ, PPARγ, ADRP, Angptl4; Fig. 7).
Effects of fish oil (FO) and Tamoxifen (TAM) on gene expression in tumors. a Estrogen responsive genes. b Peroxisome proliferator-responsive genes. Bars represent means ± SE. The numbers above the bars represent the n. See “Materials and Methods” section for experimental details. ADRP adipose differentiation-related protein, Angptl4 angiopoeitin-like protein 4/fasting-induced adipose factor, AREG amphiregulin, FGF fibrinogen beta chain, GAS6 growth arrest-specific 6, KRT19 keratin 19, PGR progesterone receptor, PPARα peroxisome proliferator-activated receptor alpha, PPARβ/δ peroxisome proliferator-activated receptor beta/delta, PPARγ peroxisome proliferator-activated receptor gamma, SCD1 stearoyl-coenzyme A desaturase 1
Discussion
Optimal breast cancer prevention is likely to require a safe multi-targeted approach as mammary carcinogenesis requires the coordinated activation of multiple cellular events resulting from complex interactions between genetic and environmental factors.
The prevention of hormone-independent tumors remains a particularly challenging problem as there are currently no known effective regimens able to prevent the development of this aggressive tumor phenotype. We hypothesize that ER, which are frequently upregulated in preneoplastic mammary lesions [1], may contribute to the development of hormone-independent tumors through bidirectional crosstalk with multiple cellular oncogenic pathways. To test this hypothesis, we evaluated the chemopreventive efficacy of the combination of the antiestrogen Tamoxifen with FO administration. Most experimental studies in rodents have shown that diets high in omega-3 fatty acids found in FO inhibit mammary carcinogenesis [20]. Although the specific mechanisms of the antitumor effects of FO remain to be elucidated, they are likely to influence multiple pathways involved in carcinogenesis (see ref. [21] for review). Pertinent to our study is the fact that some of these pathways, such as the PI-3K pathway, have been shown to be synergistically downregulated by TAM and FO in breast cancer cells [7]. This finding, together with the excellent safety profile of FO, makes this combination translationally attractive to test for its ability to inhibit the development of hormone-independent mammary tumors. We chose to conduct our studies in polyoma middle T transgenic mice, since in this experimental system the development of hormone-independent tumors is preceded by preneoplastic lesions that overexpress functional ER as evidenced by the concomitant upregulation of progesterone receptors [11] which are products of estrogen action. Other investigators have observed that even at the stage of adenocarcinomas, polyoma middle T mammary tumors retain the expression of ER and sensitivity to the growth-promoting effects of physiologic doses of estradiol in vivo [22].
Our results clearly show that Tamoxifen administration significantly delayed mammary carcinogenesis and reduced tumor multiplicity and volume in a dose-dependent fashion. Ovariectomy has also been shown by Rondinelli et al. [23] to retard polyomavirus mammary tumorigenesis. This finding points to the likely importance of the presence of ER in preneoplastic lesions for the development of hormone-independent tumors. A chemopreventive effect of Tamoxifen has also been reported in other transgenic models of hormone-independent tumors [24–26], although the ER status of the premalignant lesions in these models has not been described. In contrast, Tamoxifen did not inhibit the growth of established tumors (Fig. 4), a finding consistent with the loss of ER expression after progression of the premalignant lesions to invasive adenocarcinomas as reported by Lin et al. [11]. Accordingly, Tamoxifen did not affect the expression of estrogen related genes in established tumors at termination (Fig. 7a). It would be of interest to test the effects of Tamoxifen on the expression of estrogen related genes by preneoplastic ER positive lesions. Although Tamoxifen administration significantly delayed carcinogenesis, nearly all mice ultimately developed mammary cancer. This finding further emphasizes that targeting the ER alone is not sufficient to prevent the development of hormone-independent tumors. It does indicate, however, that antiestrogens may be effective to achieve this goal if used in combination with other mechanistically based selected chemopreventive agents.
In our experiments, FO did not demonstrate a chemopreventive effect either when used alone at concentrations ranging from 5% to 17% or in combination with TAM. Consistent with this finding is the lack of effect of FO-rich diets on the expression of PPAR-related genes (Fig. 7b). In previous studies conducted in a prepubertal model of MNU-induced rat mammary carcinogenesis, we observed that a 17% FO-rich diet, while having no effect on its own potentiated the chemopreventive action of Tamoxifen [6]. In addition, in the present experiments we tested a diet with equal amounts of FO and CO (10% each) since some investigators have suggested that an n-3/n-6 ratio between 0.5 and 1 is critical for the chemoprotective effect of FO on mammary carcinogenesis [18, 19]. However, neither 10% nor 17% FO potentiated the antitumor action of Tamoxifen or had any effect of its own in this polyoma middle T transgenic model.
A strength of our studies is that we experimentally verified the FA acid composition of the diets as well as that of the plasma and tumors of the mice. Such detailed comprehensive analysis is essential in order to determine the actual (instead of calculated) n-3/n-6 ratio in the diet and to appreciate the influence of systemic and tissue specific factors affecting FA metabolism. This point is highlighted by the FA composition of the plasma and tumors of the mice fed the 17% FO diet where the expected further rise in n-3 levels compared to the mice fed the 10% diet was not observed. This finding, which could not be explained by errors in diet preparation, points to the possible influence of some host related factors, systemic and/or tissue specific, on FA metabolism in response to the increasing amount of FO in the diet. Such modifications in FA metabolism may be critical in determining the antitumor action (or lack of it) of FO and can only be appreciated if detailed FA analysis of the diets, plasma, and target tissue are performed.
One of the postulated mechanisms of the antitumor effect of FO is through suppression of arachidonic acid (AA) derived pro-inflammatory and tumor promoting eicosanoids such as PGF-2α [21]. We observed that FO, while able to reduce in a dose-dependent fashion the tumor levels of PGF-2α, was not able to prevent mammary carcinogenesis. A similar finding has also been reported by other investigators [27]. These observations suggest that, at least in some experimental systems, suppression of AA metabolism by FO is not sufficient to inhibit mammary carcinogenesis.
It should be emphasized that the lack of chemopreventive efficacy of FO reported here is restricted to this experimental model and should not be generalized to breast cancer prevention in general. Yee et al. [28] observed a major chemopreventive effect of an FO-rich diet in the HER-2/neu transgenic model of mammary carcinogenesis. In their experiments, only 25% of caloric intake was derived from fat as opposed to nearly 40% in our studies. This may be a critical experimental variable explaining the difference in our results since some investigators have suggested that FO inhibits mammary carcinogenesis when given with a low fat but not with a high fat diet [29]. Alternatively, or in addition, the carcinogenic steps activated by HER-2neu overexpression may be differentially affected by FO compared to those activated in the polyoma middle T model. Testing the effects of FO simultaneously in the two systems under identical experimental conditions and including a low fat diet may allow to gain insight into the influence of gene/diet interactions on mammary carcinogenesis. These studies are ongoing in our laboratories.
References
Allred DC, Mohsin SK, Fuqua SA (2001) Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer 8:47–61
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90:1371–1388
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC (1999) The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. Jama 281:2189–2197
Osborne CK, Shou J, Massarweh S, Schiff R (2005) Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 11:865s–870s
Johnston SR (2005) Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res 11:889s–899s
Manni A, Xu H, Washington S, Aliaga C, Cooper T, Richie JP Jr, Bruggeman R, Prokopczyk B, Calcagnotto A, Trushin N, Mauger D, Verderame MF, El-Bayoumy K (2010) The impact of fish oil on the chemopreventive efficacy of tamoxifen against development of N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Cancer Prev Res 3:322–330
Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Ando S (2005) Estrogen receptor alpha binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor gamma signaling in breast cancer cells. Clin Cancer Res 11:6139–6147
Wang X, Kilgore MW (2002) Signal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol 194:123–133
MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim YW, Traina SB, Hilton L, Garland R, Morton SC (2006) Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA 295:403–415
Ruxton CH, Reed SC, Simpson MJ, Millington KJ (2007) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 20:275–285
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW (2003) Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 163:2113–2126
Guy CT, Cardiff RD, Muller WJ (1992) Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12:954–961
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27:114–120
Gopinathan L, Hannon DB, Smith Iii RW, Peters JM, Vanden Heuvel JP (2008) Regulation of peroxisome proliferator-activated receptors by e6-associated protein. PPAR Res 2008:746935
Wallen WJ, Belanger MP, Wittnich C (2001) Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr 131:2351–2357
Wallen WJ, Belanger MP, Wittnich C (2002) Body weight and food intake profiles are modulated by sex hormones and tamoxifen in chronically hypertensive rats. J Nutr 132:2246–2250
Greaves P, Goonetilleke R, Nunn G, Topham J, Orton T (1993) Two-year carcinogenicity study of tamoxifen in Alderley Park Wistar-derived rats. Cancer Res 53:3919–3924
Cohen LA, Chen-Backlund JY, Sepkovic DW, Sugie S (1993) Effect of varying proportions of dietary menhaden and corn oil on experimental rat mammary tumor promotion. Lipids 28:449–456
Wei N, Wang B, Zhang QY, Mi MT, Zhu JD, Yu XP, Yuan JL, Chen K, Wang J, Chang H (2008) Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr Cancer 60:810–825
Edwards IJ, Berquin IM, Yong QC, O'Flaherty JT (2010) w-3 PUFAs, breast and prostate cancer: experimental studies. In: Calviello G, Serini S (eds) Dietary omega-3 polyunsaturated fatty acids and cancer. Springer, Berlin, pp 167–188
Sawyer MB, Field CJ (2010) Possible mechanisms of w-3 PUFA anti-tumor action. In: Calviello G, Serini S (eds) Dietary omega-3 polyunsaturated fatty acids and cancer. Springer, Berlin, pp 3–38
Dabrosin C, Palmer K, Muller WJ, Gauldie J (2003) Estradiol promotes growth and angiogenesis in polyoma middle T transgenic mouse mammary tumor explants. Breast Cancer Res Treat 78:1–6
Rondinelli RH, Haslam SZ, Fluck MM (1995) The role of ovarian hormones, age and mammary gland development in polyomavirus mammary tumorigenesis. Oncogene 11:1817–1827
Menard S, Aiello P, Tagliabue E, Rumio C, Lollini PL, Colnaghi MI, Balsari A (2000) Tamoxifen chemoprevention of a hormone-independent tumor in the proto-neu transgenic mice model. Cancer Res 60:273–275
Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Iezzi M, Ricci C, Astolfi A, Croci S, Marangoni F, Musiani P, Forni G, Lollini PL (2003) Prevention of HER-2/neu transgenic mammary carcinoma by tamoxifen plus interleukin 12. Int J Cancer 105:384–389
Medina D, Kittrell FS, Hill J, Shepard A, Thordarson G, Brown P (2005) Tamoxifen inhibition of estrogen receptor-alpha-negative mouse mammary tumorigenesis. Cancer Res 65:3493–3496
Sasaki T, Kobayashi Y, Shimizu J, Wada M, In'nami S, Kanke Y, Takita T (1998) Effects of dietary n-3-to-n-6 polyunsaturated fatty acid ratio on mammary carcinogenesis in rats. Nutr Cancer 30:137–143
Yee LD, Young DC, Rosol TJ, Vanbuskirk AM, Clinton SK (2005) Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARgamma ligand rosiglitazone. J Nutr 135:983–988
Olivo SE, Hilakivi-Clarke L (2005) Opposing effects of prepubertal low- and high-fat n-3 polyunsaturated fatty acid diets on rat mammary tumorigenesis. Carcinogenesis 26:1563–1572
Acknowledgments
This work is supported by grant KG081632 from Susan G. Komen for the Cure.
Conflict of Interest
None of the authors have any information to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Composition of experimental AIN-76A diets (DOC 40 kb)
Supplementary Table 2
Fatty acid analysis of fish oil rich dietsa (DOC 48 kb)
Supplementary Table 3
Primer sequences (DOC 35 kb)
Supplementary Table 4
Effect of fish oil on plasma and tumor fatty acid concentrations (DOC 86 kb)
Rights and permissions
About this article
Cite this article
Manni, A., Xu, H., Washington, S. et al. The Effects of Tamoxifen and Fish Oil on Mammary Carcinogenesis in Polyoma Middle T Transgenic Mice. HORM CANC 2, 249–259 (2011). https://doi.org/10.1007/s12672-011-0078-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-011-0078-2