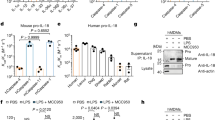
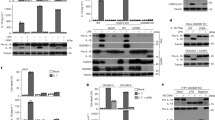
Abstract
The interleukin (IL)-1 family is the largest family of interleukins. Eleven members of the IL-1 family of ligands are intracellular molecules, except a single isoform of an IL-1 receptor antagonist (IL-1Ra; also known as IL-1RN), which contains a signal peptide at the N-terminus for effective secretion. The inflammasome is a complex of intracellular molecules that is responsible for the processing of IL-1β and IL-18, whereas the remaining IL-1 family members, including IL-1α, are processed in an inflammasome caspase-1-independent pathway. Among the eleven members of the IL-1 family ligands, precursor IL-1α, IL-1β, and IL-33 have comparatively long pro-peptides of approximately 110 amino acid residues at the N-terminus. However, the other IL-1 members, except for IL-37 (also known as IL-1F7), have relatively short propeptides with fewer than 40 amino acid residues at the N-terminus. Most cytokines, including interferons and interleukins, possess a hydrophobic signal sequence for secretion. Therefore, soluble cytokines readily act on cell surface receptors immediately after their release from cells. Unlike other cytokine families, IL-1 family ligands exhibit two-step regulation: transcriptional induction at the mRNA level and post-translational modification at the protein level because of the lack of a hydrophobic signal sequence at the N-terminus. Various processing enzymes involved in the activation of intracellular IL-1 family cytokines likely provide effective immune regulation to protect the host from infections. In this review, we describe all eleven IL-1 family ligand processing enzymes, mature ligand functions, and mode of receptor conformation.




Similar content being viewed by others
References
Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Luthi AU, Reeves EP, McElvaney NG, Medema JP, Lavelle EC, Martin SJ (2011) Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol Cell 44:265–278
Ahn HJ, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H (1997) A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol 159:2125–2131
Arend WP, Palmer G, Gabay C (2008) IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 223:20–38
Azam T, Novick D, Bufler P, Yoon DY, Rubinstein M, Dinarello CA, Kim SH (2003) Identification of a critical Ig-like domain in IL-18 receptor alpha and characterization of a functional IL-18 receptor complex. J Immunol 171:6574–6580
Bae S, Kang T, Hong J, Lee S, Choi J, Jhun H, Kwak A, Hong K, Kim E, Jo S, Kim S (2012) Contradictory functions (activation/termination) of neutrophil proteinase 3 enzyme (PR3) in interleukin-33 biological activity. J Biol Chem 287:8205–8213
Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, Lohning M (2015) T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci USA 112:4056–4061
Blom L, Poulsen LK (2012) IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures. J Immunol 189:4331–4337
Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, Fitzgerald KA, Marshak-Rothstein A, Latz E (2012) Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol 189:5508–5512
Brandolini L, Bertini R, Bizzarri C, Sergi R, Caselli G, Zhou D, Locati M, Sozzani S (1996) IL-1 beta primes IL-8-activated human neutrophils for elastase release, phospholipase D activity, and calcium flux. J Leukoc Biol 59:427–434
Bufler P, Azam T, Gamboni-Robertson F, Reznikov LL, Kumar S, Dinarello CA, Kim SH (2002) A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA 99:13723–13728
Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA (2004) Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J 381:503–510
Bulau AM, Nold MF, Li S, Nold-Petry CA, Fink M, Mansell A, Schwerd T, Hong J, Rubartelli A, Dinarello CA, Bufler P (2014) Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA 111:2650–2655
Cannon JG, Dinarello CA (1985) Increased plasma interleukin-1 activity in women after ovulation. Science 227:1247–1249
Carroll TP, Greene CM, Taggart CC, Bowie AG, O’Neill SJ, McElvaney NG (2005) Viral inhibition of IL-1- and neutrophil elastase-induced inflammatory responses in bronchial epithelial cells. J Immunol 175:7594–7601
Cayrol C, Girard JP (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA 106:9021–9026
Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256:97–100
Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, Leimer AH, Cheronis J (1999) Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA 96:6261–6266
Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 261:472–475
Darmon AJ, Ehrman N, Caputo A, Fujinaga J, Bleackley RC (1994) The cytotoxic T cell proteinase granzyme B does not activate interleukin-1 beta-converting enzyme. J Biol Chem 269:32043–32046
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732
Dinarello CA, Bufler P (2013) Interleukin-37. Semin Immunol 25:466–468
Dunne A, Ejdeback M, Ludidi PL, O’Neill LA, Gay NJ (2003) Structural complementarity of Toll/interleukin-1 receptor domains in Toll-like receptors and the adaptors Mal and MyD88. J Biol Chem 278:41443–41451
Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, Thompson RC (1990) Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature 343:341–346
Engelmann H, Novick D, Wallach D (1990) Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem 265:1531–1536
Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H (1997) Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619–623
Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS (1997) Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206–209
Hardy MP, Mc GAF, O’Neill LA (2004) Transcriptional regulation of the human TRIF (TIR domain-containing adaptor protein inducing interferon beta) gene. Biochem J 380:83–93
Haskill S, Martin G, Van Le L, Morris J, Peace A, Bigler CF, Jaffe GJ, Hammerberg C, Sporn SA, Fong S (1991) cDNA cloning of an intracellular form of the human interleukin 1 receptor antagonist associated with epithelium. Proc Natl Acad Sci USA 88:3681–3685
Hayakawa M, Hayakawa H, Matsuyama Y, Tamemoto H, Okazaki H, Tominaga S (2009) Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem Biophys Res Commun 387:218–222
Helmby H, Grencis RK (2002) IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-gamma during Trichinella spiralis infection. J Immunol 169:2553–2560
Henry CM, Sullivan GP, Clancy DM, Afonina IS, Kulms D, Martin SJ (2016) Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep 14:708–722
Hong J, Bae S, Jhun H, Lee S, Choi J, Kang T, Kwak A, Hong K, Kim E, Jo S, Kim S (2011) Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem 286:20078–20086
Hoshino T, Yagita H, Ortaldo JR, Wiltrout RH, Young HA (2000) In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol 30:1998–2006
Irmler M, Hertig S, MacDonald HR, Sadoul R, Becherer JD, Proudfoot A, Solari R, Tschopp J (1995) Granzyme A is an interleukin 1 beta-converting enzyme. J Exp Med 181:1917–1922
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121
Kim SH, Cohen B, Novick D, Rubinstein M (1997) Mammalian type I interferon receptors consists of two subunits: IFNaR1 and IFNaR2. Gene 196:279–286
Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello CA (2000) Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA 97:1190–1195
Kim SH, Azam T, Yoon DY, Reznikov LL, Novick D, Rubinstein M, Dinarello CA (2001a) Site-specific mutations in the mature form of human IL-18 with enhanced biological activity and decreased neutralization by IL-18 binding protein. Proc Natl Acad Sci USA 98:3304–3309
Kim SH, Reznikov LL, Stuyt RJ, Selzman CH, Fantuzzi G, Hoshino T, Young HA, Dinarello CA (2001b) Functional reconstitution and regulation of IL-18 activity by the IL-18R beta chain. J Immunol 166:148–154
Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA (2013) The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol 4:391
Kroeger KM, Sullivan BM, Locksley RM (2009) IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol 86:769–778
Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S, Kirkpatrick RB, Scott GF, Lee JC, Lynch FJ, Gao W, Gambotto A, Lotze MT (2002) Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 18:61–71
Lee S, Kim E, Jhun H, Hong J, Kwak A, Jo S, Bae S, Lee J, Kim B, Youn S, Kim S, Kim M, Kim H, Lee Y, Choi DK, Kim YS, Kim S (2016) Proinsulin Shares a Motif with Interleukin-1alpha (IL-1alpha) and Induces Inflammatory Cytokine via Interleukin-1 Receptor 1. J Biol Chem 291:14620–14627
Lefrancais E, Cayrol C (2012) Mechanisms of IL-33 processing and secretion: differences and similarities between IL-1 family members. Eur Cytokine Netw 23:120–127
Leite-De-Moraes MC, Hameg A, Pacilio M, Koezuka Y, Taniguchi M, Van Kaer L, Schneider E, Dy M, Herbelin A (2001) IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J Immunol 166:945–951
Liu B, Novick D, Kim SH, Rubinstein M (2000) Production of a biologically active human interleukin 18 requires its prior synthesis as PRO-IL-18. Cytokine 12:1519–1525
Lomedico PT, Gubler U, Hellmann CP, Dukovich M, Giri JG, Pan YC, Collier K, Semionow R, Chua AO, Mizel SB (1984) Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature 312:458–462
Luthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ (2009) Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31:84–98
Malinowsky D, Lundkvist J, Laye S, Bartfai T (1998) Interleukin-1 receptor accessory protein interacts with the type II interleukin-1 receptor. FEBS Lett 429:299–302
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426
McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, Grubin CE, Wignall JM, Jenkins NA, Brannan CI (1991) A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J 10:2821–2832
Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M (1999) Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 10:127–136
Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S (2009) Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol 183:7890–7897
Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K (1995) Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378:88–91
Palomo J, Dietrich D, Martin P, Palmer G, Gabay C (2015) The interleukin (IL)-1 cytokine family–balance between agonists and antagonists in inflammatory diseases. Cytokine 76:25–37
Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT (2010) Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA 107:8017–8022
Radons J, Dove S, Neumann D, Altmann R, Botzki A, Martin MU, Falk W (2003) The interleukin 1 (IL-1) receptor accessory protein Toll/IL-1 receptor domain: analysis of putative interaction sites in vitro mutagenesis and molecular modeling. J Biol Chem 278:49145–49153
Scala G, Allavena P, Djeu JY, Kasahara T, Ortaldo JR, Herberman RB, Oppenheim JJ (1984) Human large granular lymphocytes are potent producers of interleukin-1. Nature 309:56–59
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479–490
Shi L, Chen G, MacDonald G, Bergeron L, Li H, Miura M, Rotello RJ, Miller DK, Li P, Seshadri T, Yuan J, Greenberg AH (1996) Activation of an interleukin 1 converting enzyme-dependent apoptosis pathway by granzyme B. Proc Natl Acad Sci USA 93:11002–11007
Sims JE, March CJ, Cosman D, Widmer MB, MacDonald HR, McMahan CJ, Grubin CE, Wignall JM, Jackson JL, Call SM (1988) cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science 241:585–589
Smeltz RB, Chen J, Hu-Li J, Shevach EM (2001) Regulation of interleukin (IL)-18 receptor alpha chain expression on CD4(+) T cells during T helper (Th)1/Th2 differentiation. Critical downregulatory role of IL-4. J Exp Med 194:143–153
Smith DE, Hanna R, Della F, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE (2003) The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity 18:87–96
Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE (2008) IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 20:1019–1030
Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem 277:21119–21122
Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takada H (2001) Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol 167:6568–6575
Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G (2009) Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem 284:19420–19426
Thornberry NA, Bull HG, Calaycay J, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774
Towne JE, Renshaw BR, Douangpanya J, Lipsky BP, Shen M, Gabel CA, Sims JE (2011) Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36alpha, IL-36beta, and IL-36gamma) or antagonist (IL-36Ra) activity. J Biol Chem 286:42594–42602
Uchida T, Kinoshita M, Fukasawa M, Habu Y, Shinomiya N, Seki S (2007) IL-18 time-dependently modulates Th1/Th2 cytokine production by ligand-activated NKT cells. Eur J Immunol 37:966–977
Van Damme J, De Ley M, Opdenakker G, Billiau A, De Somer P, Van Beeumen J (1985) Homogeneous interferon-inducing 22K factor is related to endogenous pyrogen and interleukin-1. Nature 314:266–268
van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V, Dinarello CA (2012) IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci 109:3001–3005
Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin MU (1997) The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases). J Biol Chem 272:7727–7731
Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S (2000) IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol 164:2701–2710
Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, Gracie JA, McInnes IB, Liew FY (2000) IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol 30:3147–3156
Acknowledgements
This paper was supported by Konkuk University in 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Areum Kwak and Youngmin Lee have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kwak, A., Lee, Y., Kim, H. et al. Intracellular interleukin (IL)-1 family cytokine processing enzyme. Arch. Pharm. Res. 39, 1556–1564 (2016). https://doi.org/10.1007/s12272-016-0855-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0855-0