Abstract
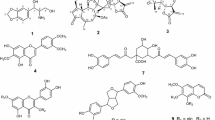
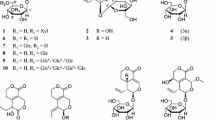
A new sinapoyl glycoside, 1,3-di-O-sinapoyl-β-d-glucopyranose (1) along with 13 known compounds, including, sinapoyl glycosides (2 and 3), cardenolide glycoside (4), flavonoids (5–10), lignan (11), phenolic acids (12 and 13), and phytosterol (14), were isolated from the seeds of Descurainia sophia by chromatographic separation methods. The structures of 1–14 were determined by the interpretation of spectroscopic data as well as by comparison of that data with previously reported values. Compounds 2, 3, 5, 6, and 11 were identified in and isolated from this plant for the first time in this study. All isolates were evaluated for in vitro cytotoxic activities against seven human cancer cell lines and for in vitro anti-inflammatory potential using LPS-stimulated RAW264.7 murine macrophages. Compound 4 showed potent cytotoxicity (IC50 values ranging from 0.034 to 0.596 μM) against all human cancer cell lines tested and was identified as the main active cytotoxic constituent of this plant. Compound 8 (ED50 = 5.45 μM) and 11 (ED50 = 10.02 μM) exerted dose-dependent inhibitory effects on NO production in LPS-stimulated RAW264.7 cells.



Similar content being viewed by others
References
Bekker, N.P., N.T. Ul’chenko, and A.I. Glushenkova. 2005. Lipids from Descurainia Sophia seeds. Chemistry of Natural Compounds 41: 346–347.
Chen, Y.Q., R.Z. Li, and Y.W. Wang. 1981. Identification of cardiac glycosides from the seeds of Descurainia sophia L. Webb. Acta Pharmaceutica Sinica 16: 62–64.
Choi, J., M. Jung, H. Park, H. Chung, and S. Kang. 2002. Further isolation of peroxynitrite and 1,1-diphenyl-2-picrylhy-drazyl radical scavenging isorhamnetin 7-O-glucoside from the leaves of Brassica juncea L. Archives of Pharmacal Research 25: 625–627.
Dajas, F. 2012. Life or death: neuroprotective and anticancer effects of quercetin. Journal of Ethnopharmacology 143: 383–396.
Geiger, H., H. Maier, and K.R. Markham. 1983. Quercetin-3-α-L-(2-O-α-L-rhamnopyranosido-arabopyranosid), ein neues flavonolglykosid aus den Samen von Brassica nigra (L.) Koch. Zeitschrift für Naturforschung 38: 490–491.
Kim, A.R., J.Y. Cho, Y. Zou, J.S. Choi, and H.Y. Chung. 2005. Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW264.7 cells. Archives of Pharmacal Research 28: 297–304.
Kim, H.Y., B.H. Moon, H.J. Lee, and D.H. Choi. 2004. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. Journal of Ethnopharmacology 93: 227–230.
Kim, K.H., S.K. Ha, S.Y. Kim, H.J. Youn, and K.R. Lee. 2010. Constituents of Limonia acidissima inhibit LPS-induced nitric oxide production in BV-2 microglia. Journal of Enzyme Inhibition and Medicinal Chemistry 25: 887–892.
Ko, J.H., B.G. Kim, J.H. Kim, H. Kim, C.E. Lim, J. Lim, C. Lee, Y. Lim, and J.H. Ahn. 2008. Four glucosyltransferases from rice: cDNA cloning, expression, and characterization. Journal of Plant Physiology 165: 435–444.
Kopp, B., L. Krenn, E. Kubelka, and W. Kubelka. 1992. Cardenolides from Adonis aestivalis. Phytochemistry 31: 3195–3198.
Kuo, W.-L., Y.-L. Huang, S.-T. Wang, C.-L. Ni, B.-J. Shien, and C.-C. Chen. 2007. Chemical constituents of Trema orientalis. Journal of Chinese Medicine 18: 27–36.
Lee, S., K.S. Kim, J.M. Jang, Y. Park, Y.B. Kim, and B.K. Kim. 2002. Phytochemical constituents from the herba of Artemisia apiacea. Archives of Pharmacal Research 25: 285–288.
Lei, Z.H., S. Yahara, T. Nohara, T.B. Shan, and J.Z. Xiong. 1996. Cardenolides from Erysimum cheiranthoides. Phytochemistry 41: 1187–1189.
Lim, S.S., Y.J. Jung, S.K. Hyun, Y.S. Lee, and J.S. Choi. 2006. Rat lens aldose reductase inhibitory constituents of Nelumbo nucifera stamens. Phytotherapy Research 20: 825–830.
Mohamed, N.H., and A.E. Mahrous. 2009. Chemical constituents of Descurainia sophia L. and its biological activity. Records of Natural Products 3: 58–67.
Moon, S.S., M.A. Rahman, M.M. Manir, and V.S. Jamal Ahamed. 2010. Kaempferol glycosides and cardenolide glycosides, cytotoxic constituents from the seeds of Draba nemorosa (Brassicaceae). Archives of Pharmacal Research 33: 1169–1173.
Muhammad, K., Y. Xiao, Y. Bo, W. Nan, R. Azhar, Y. Fei, Y. Longfei, Y. Hong, Y. Hong, and M. Tonghui. 2012. Artabotryside A, a constituent from Descurainia sophia (L.) induces cell death in U87 glioma cells through apoptosis and cell cycle arrest at G2/M phase. Journal of Medicinal Plants Research 6: 3754–3765.
Nakamura, T., Y. Goda, S. Sakai, K. Kondo, H. Akiyama, and M. Toyoda. 1998. Cardenolide glycosides from seeds of Corchorus olitorius. Phytochemistry 49: 2097–2101.
Price, K.R., F. Casuscelli, I.J. Colquhoun, and M.J.C. Rhodes. 1997. Hydroxycinnamic acid esters from broccoli florets. Phytochemistry 45: 1683–1687.
Rhaman, M.A.A., and S.-S. Moon. 2007. Antioxidant polyphenol glycosides from the plant Daraba nemorosa. Bulletin of the Korean Chemical Society 28: 827–831.
Rubino, M.I., S.D. Arntfield, and J.L. Charlton. 1995. Conversion of phenolics to lignans: sinapic acid to thomasidioic acid. Journal of the American Oil Chemists Society 72: 1465–1470.
Rubino, M.I., S.D. Arntfield, and J.L. Charlton. 1996. Evaluation of alkaline conversion of sinapic acid to thomasidioic acid. Journal of Agriculture and Food Chemistry 44: 1399–1402.
Sun, K., X. Li, W. Li, J.M. Liu, J.H. Wang, and Y. Sha. 2006. A new nor-lignan from the seeds of Descurainia sophia. Natural Product Research 20: 519–522.
Sun, K., X. Li, W. Li, J. Wang, J. Liu, and Y. Sha. 2004. Two new lactones and one new aryl-8-oxa-bicyclo[3,2,1]oct-3-en-2-one from Descurainia sophia. Chemical & Pharmaceutical Bulletin 52: 1483–1486.
Sun, K., X. Li, J.M. Liu, J.H. Wang, W. Li, and Y. Sha. 2005. A novel sulphur glycoside from the seeds of Descurainia sophia (L.). Journal of Asian Natural Products Research 7: 853–856.
Takaya, Y., Y. Kondo, T. Furukawa, and M. Niwa. 2003. Antioxidant constituents of radish sprout (Kaiware-daikon), Raphanus sativus L. Journal of Agriculture and Food Chemistry 51: 8061–8066.
Wang, A.Q., X.K. Wang, J.L. Li, and X.Y. Cui. 2004. Isolation and structure identification of chemical constituents from the seeds of Descurainia sophia (L.) Webb ex Prantl. Acta Pharmaceutica Sinica B 39: 46–51.
Acknowledgments
This research was supported by grant [K11062] from the Korea Institute of Oriental Medicine. We thank the Korea Basic Science Institute (KBSI) for performing the NMR and MS experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
You Jin Lee and No Soo Kim contributed equally to this study.
Rights and permissions
About this article
Cite this article
Lee, Y.J., Kim, N.S., Kim, H. et al. Cytotoxic and anti-inflammatory constituents from the seeds of Descurainia sophia . Arch. Pharm. Res. 36, 536–541 (2013). https://doi.org/10.1007/s12272-013-0066-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0066-x