Abstract
Pleural and peritoneal infections cause substantial morbidity and mortality. Traditional diagnostic methods rely on the cultivation of clinical samples, which usually takes days to obtain report and holds a low detection sensitivity. In this study, we evaluated a 5-fluorescent-channel droplet digital PCR (ddPCR) system and 5 assay panels for culture-independent rapid pathogen detections directly from pleural and peritoneal fluid samples. Traditional culture of the same sample was used as reference. A total of 40 pleural fluid samples and 19 peritoneal fluid samples were tested in this study. Twenty-five positives including 4 polymicrobial infections by culture and 26 positives including 11 polymicrobial infections by ddPCR were detected for pleural fluid samples; 14 positives including 2 polymicrobial infections by culture and 15 positives including 3 polymicrobial infections by ddPCR were detected for peritoneal fluid samples. Klebsiella pneumoniae was the most common bacterium detected both in pleural and in peritoneal fluid samples. The sensitivity of the ddPCR assay for pleural and peritoneal fluid samples was 96% (95% confidence interval (CI) = 79.65 to 99.90%) and 92.86% (95% CI = 66.13 to 99.82%), respectively. The turnaround time of the ddPCR assay was approximately 3 h comparing with 38.30 ± 22.44 h for culture-based identifications. Our results demonstrated that the ddPCR assay is a rapid and sensitive method for identifying pathogens responsible for pleural and peritoneal infections and would be a promising approach for early diagnosis and optimizing treatment of infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pleural and peritoneal infections are common and important clinical problems worldwide. The incidence in both adult and pediatric populations continues to rise and causes substantial morbidity and mortality, with up to 20% of patients requiring surgery or dying (Corcoran et al. 2015). Timely and appropriate antibiotic treatment can improve the clinical outcome; however, inappropriate use and delayed initiation of antibiotics remain common. Identification of the causative organisms involved in pleural and peritoneal infections is important and particularly useful to guide antimicrobial therapy (Maskell et al. 2006; Picazo et al. 2013).
Currently, bacterial culture is the gold standard method for identification of pathogens in clinical samples. However, the detection rates are as low as between 17 and 42% for pleural fluid cultures (Jiménez et al. 2006). Almost half of infected pleural effusions turn out to be microbiologically negative (Maskell et al. 2006). Fastidious and slowly growing microorganisms or broad-spectrum antimicrobial treatment before obtaining pleural/peritoneal fluids for culture often leads to false-negative results. Moreover, bacterial culture usually takes 1 to 5 or more days (She et al. 2018). For highly suspected patients with infections, the shorter the diagnostic procedure, the better the clinical outcome may be. Therefore, a rapid and accurate diagnostic method for early detection of the causative organisms in clinical samples remains an urgent clinical need and an important research direction.
Cell-free DNA (cfDNA) has emerged in the past decade as a promising biomarker for noninvasive testing in oncology and obstetrics (Bianchi et al. 2014; Crowley et al. 2013; Wan et al. 2017). In the field of infectious diseases, pathogen cfDNA has also been applied for the diagnosis of tuberculosis (Click et al. 2018; Li et al. 2019; Ushio et al. 2016), invasive fungal infections (Wang et al. 2017; White et al. 2015), and parasitic infections (Weerakoon and McManus 2016). However, most of the samples were blood or urine specimens. Pathogen cfDNA in pleural and peritoneal fluid has not been fully explored in this regard.
Polymerase chain reaction (PCR)–based assays, such as multiplex PCR and real-time quantitative PCR (qPCR), have been used to detect microorganisms in pleural and peritoneal infections and have showed potential for clinical diagnosis (Amin et al. 2019; Hardick et al. 2012; Wu et al. 2015). Digital PCR (dPCR) is a new approach to nucleic acid detection and quantification with higher accuracy and sensitivity than qPCR. Digital PCR are performed by partitioning the sample reaction mixture into a large number of separate reactions in a small volume. Each individual reaction contains single, few, or no target sequences. Then, these small reactions are amplified individually. After the amplification, reactions containing target sequence are detected by fluorescence and scored as positive, and reactions without fluorescence are scored as negative. Poisson statistical analysis of the numbers of positive and negative reactions yields absolute quantitation of the target sequence, without the need of calibrators and standard curves, solving some shortcomings of qPCR (Vogelstein and Kinzler 1999). Partitioning renders PCR less sensitive to reaction inhibitors, and reduces any template competition, which improves the multiplexing capability of dPCR. These characteristics make dPCR a promising sensitive method for pathogen detection of infectious disease. The aim of this study was to evaluate a digital PCR method on pathogen detection in pleural and peritoneal fluid samples, and determine the sensitivity and concordance with species identification against standard culture techniques.
Materials and methods
Study design
This study was approved by the Medical Ethics Committee of the University (No. 2019-KL-090-01) and was conducted in compliance with ethical, legal, and regulatory norms. Written consent was waived as this study used saved pleural and peritoneal fluid samples and only retrospectively reviewed and analyzed the clinical data of patients, and there was no need for patients to provide additional specimens.
Experiments were conducted in the Department of Clinical Laboratory, the First Affiliated Hospital of Zhejiang Chinese Medical University from July 2019 to January 2020. A total of 40 pleural fluid samples and 19 peritoneal samples from patients who were clinically suspected to have pleural or peritoneal infections were evaluated by the conventional culture and ddPCR method.
Microbiological culture
The pleural and peritoneal fluid samples were cultured under aerobic and anaerobic conditions for up to 5 days or until positive signal appeared. The bacterial isolates were identified at species level using Microflex LT/SH (Bruker) matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Nucleic acid extraction
Pleural and peritoneal fluid samples were centrifuged at 1500g for 15 min at 4 °C. Two milliliters of supernatant plus 10 μl of internal control was transferred to an Auto-Pure20B nucleic acid purification system (Hangzhou Allsheng Instruments Co., Ltd., Hangzhou, China) for cell-free DNA (cfDNA) isolation using a Magnetic Serum/Plasma DNA Kit (Tiangen Biotech, Beijing, China) according to the manufacturers’ instructions. The cfDNA was eluted into 50 μl of 10 mM Tri-EDTA buffer. The internal control was a randomly generated and then synthesized 200-bp DNA fragment to monitor extraction process.
Droplet digital PCR analysis
Droplet digital PCR (ddPCR) analysis was performed using a 5-fluorescent-channel droplet digital PCR system (Pilot Gene Technology (Hangzhou) Co., Ltd., Hangzhou, China). Four PilotBac assay panels and 1 PilotFungi assay panel were used in this study. Each panel can detect 4 different target pathogens besides one internal control (Table 1). Briefly, 5 μl of cfDNA template was added to 10 μl of the ddPCR premix which includes detection primers, probes, and the necessary components for PCR amplification. The reaction mixture was gently mixed and added into a ready-to-use disposable plastic chip. About 20,000 water-in-oil emulsion droplets were generated inside the chip by a droplet generator (DG32, Pilot Gene Tech.). Chips were then amplified in a thermal cycler (TC1, Pilot Gene Tech.) using the following cycling parameters: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Finally, chips were loaded into a chip scanner (iScanner 5, Pilot Gene Tech.) for fluorescence signal reading and further data analysis. According to the manufacturers’ instructions of the assay panels, the threshold for target detection was 0.7 copy/μl. A ddPCR is defined as positive if the concentration is over the threshold.
Data analysis
Analysis of the ddPCR data was performed with GenePMS v.2.0.01.20011 (Pilot Gene Tech.) to calculate the concentration of the target. The diagnostic performance of ddPCR was calculated by MEDCALC (https://www.medcalc.org/calc/diagnostic_test.php).
Results
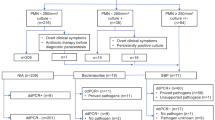
A total of 40 pleural fluid samples were analyzed in this study, among which 25 (25/40, 62.5%) were culture positive. There were 12 kinds of pathogens identified by traditional culture, including 5 gram-negative bacteria, 5 gram-positive bacteria, and 3 fungi. Klebsiella pneumonia was the most commonly detected bacterium which was reported in 7 samples (7/25, 28%). There were also 4 polymicrobial infections, including three Klebsiella pneumonia and Proteus mirabilis coinfections and one Klebsiella pneumonia and Escherichia coli coinfection. In contrast, the ddPCR analysis revealed 26 (26/40, 65%) positive events including 13 kinds of pathogens. The Klebsiella pneumonia accounted for 30.8% (8/26) of the total positive events. ddPCR detected 2.75 (11/4) times more polymicrobial infections than culture, including 7 coinfections with 2 pathogens, 3 with 3 pathogens, and 1 with 4 pathogens (Table 2). Samples of the chip analysis results are demonstrated in Fig. 1.
Scatter plots of four representative chips from three clinical samples. All five panels in Table 1 were assayed for each sample. Each panel can detect 4 different pathogens. FAM/VIC/ROX/CY5 represents four fluorescence channels for target pathogens. Only panels with positive result were showed for the three samples. a Result of assay panel PilotBac-2 for pleural fluid sample no. 6. FAM, Staphylococcus aureus. b Result of assay panel PilotFungi-1 for coinfection pleural fluid sample no. 35. FAM, Candida albicans; ROX, Candida parapsilosis. c and d Results of triple infected pleural fluid sample no. 36. c Assay panels for PilotBac-1; VIC, Escherichia coli; ROX, Klebsiella pneumonia. d Assay panels for PilotBac-2; CY5, Enterococcus faecalis
Pairwise analysis showed that 16 positive ddPCR detections (Table 2, samples 1–16) including 2 polymicrobial infections, and 13 negative ddPCR results (Table 2, samples 17–29) were in exact concordance with pleural fluid culture. For other 8 culture-positive samples (Table 2, samples 30–37), ddPCR detected one or more extra pathogens. The ddPCR method also demonstrated positive in 2 culture-negative samples (Table 2, samples 38 and 39). Staphylococcus capitis was reported to be positive only by culture in one sample (Table 2, sample 40), and Staphylococcus epidermidis was only identified by ddPCR once in another sample (Table 2, sample 34). Thus, when using pleural fluid culture result as reference, the ddPCR method had a sensitivity of 96% (95% confidence interval (CI) = 79.65 to 99.90%), and the specificity was 86.67% (95% CI = 59.54 to 98.34%). The positive predictive value (PPV) and negative predictive value (NPV) were 92.31% (95% CI = 76.71 to 97.76%) and 92.86% (95% CI = 65.35 to 98.90%), respectively (Table 3). When focusing on the occurrence number of pathogens, the ddPCR method demonstrated 13 more occurrences than culture (42 vs. 29), improving the positive detection rate by 45% (13/29).
Detection of peritoneal fluid samples showed similar results to pleural fluid samples. Out of the 19 samples, 15 (15/19, 78.9%) were ddPCR positive and 14 (14/19, 73.7%) were culture positive (Tables 4 and 5). Both methods detected 10 kinds of pathogens, including 3 gram-negative bacteria, 3 gram-positive bacteria, and 4 fungi. Klebsiella pneumonia was detected in 4 samples (4/14, 28.6%) by culture and in 6 samples (6/15, 40%) by ddPCR. There were 2 coinfections detected by culture and 3 by ddPCR. Pairwise analysis showed that 12 positive detections (Table 4, samples 1–12) and 3 negative results (Table 4, samples 13–15) by ddPCR were in accordance with culture. Besides, Escherichia coli was detected in extra by ddPCR in one sample (Table 4, sample16), for which only Klebsiella pneumonia was reported by culture; there were also two positive Klebsiella pneumonia infections detected by ddPCR in samples with negative culture results (Table 4, samples 17–18). Staphylococcus epidermidis was identified in one sample that had negative result by ddPCR (Table 4, sample 19). The ddPCR method for peritoneal fluid detection showed a sensitivity of 92.86% (95% CI = 66.13 to 99.82%), a specificity of 60.00% (95% CI = 14.66 to 94.73%), a PPV of 86.67% (95% CI = 68.75 to 95.05%), and a NPV of 75.00% (95% CI = 28.47 to 95.76%) when using the peritoneal fluid culture method as a reference (Table 5).
Discussion
Pleural and peritoneal infections were reported to cause high morbidity and mortality partly due to the low sensitivity and long turnaround time of pathogen detection by conventional cultures, though efforts have been made to improve the culture positivity (Menzies et al. 2011). A rapid and reliable detection of the etiologic agent would greatly benefit the correct and early pathogen-specific treatment.
In this study, we demonstrate that the detection of pathogen cfDNA by ddPCR is a rapid and sensitive method for detecting pleural and peritoneal infections, with sensitivity being 96% and 92.9%, respectively. Compared to other methods for the detection of pleural and peritoneal infections, the positive sensitivities of our ddPCR method are higher than those of the Curetis Unyvero P55 panel, a multiplex PCR-based assay used for pleural effusion in a clinical study (Franchetti et al. 2018). It is also slightly higher than that achieved by a gram probe real-time PCR (GRT-PCR) system, which targets the 16S rRNA gene and allows the simultaneous detection of gram-positive and gram-negative bacteria, but does not distinguish species (Wu et al. 2015). Our ddPCR assay for pleural and peritoneal infections also showed a higher or comparable performance to ddPCR in other clinical applications. Wouters et al. (2019) used ddPCR for the rapid broad-spectrum detection of bloodstream infections, showing an overall sensitivity of 80% (95% CI: 52–96%) and specificity of 87% (95% CI: 69–96%). In a study for the detection of SARS-CoV-2 in low viral load specimens, sensitivity of 94% (95% CI: 83–99%) and specificity of 100% (95% CI: 48–100%) were achieved (Suo et al. 2020).
In addition to the high sensitivity, the turnaround time (TAT) of this ddPCR method amounted to approximately 3 h, including about 30-min hands-on time, 45-min cfDNA isolation, 20-min droplet generation, 60-min ddPCR amplification, and 30-min chip reading and analysis. In contrast, traditional cultures took 38.30 ± 22.44 h for pathogen identifications in this study (data not shown). The greatly shortened TAT of this ddPCR method would undoubtedly facilitate the early diagnosis of infections and the further timely targeted antibiotic therapy.
Many types of infections are polymicrobial, involving more than one species at the infection site (Brogden and Guthmiller 2002). Due to the varied difficulties of pathogen culture and the existence of dominant strains in the culture process, culture-based detection methods often detect fewer pathogens than the actual existence. This ddPCR method is a culture-independent approach which will not be affected by the abovementioned factors. That would explain the much higher detection rates of polymicrobial infections by ddPCR than culture both in pleural and peritoneal fluid samples.
There was one culture-positive sample that not being correspondingly identified by ddPCR in both pleural and peritoneal fluid samples. Genomic DNA from these two culture isolates were extracted and re-examined using the corresponding ddPCR assay panels. Positive signals appeared correctly for both of them, thus ruling out the possibility of DNA sequence variations at probe position. Further confirmation by Sanger sequencing of the ddPCR target sequences also showed no variations at primer and probe positions. Therefore, these two positive culture results most likely came from sampling-related or culture-related contaminations, which occurs at a rate of 3–12% for blood culture (Hughes et al. 2018) though those for pleural and peritoneal fluid samples are still unclear. Based on the detecting principle, we speculate that this culture-independent, cfDNA-based detection method will furthest decrease the potential effects of sampling contaminations.
One limitation of this study is the relatively small size of samples evaluated. There were no pleural or peritoneal fluid samples infected with several ddPCR in-panel organisms such as Acinetobacter baumannii, Staphylococcus hominis, and Candida glabrata, and thus, the performance of detecting these pathogens by the ddPCR assay is not clear. Further studies with larger number of samples, especially including the microorganisms not tested in this study, would facilitate a whole evaluation of these ddPCR method as well as the assay panels.
Another limitation of this study is that ddPCR, as other PCR-based methods, cannot differentiate between cfDNA from dead or alive pathogens. However, studies have shown that, in the human body, the half-life of microbial cfDNA in plasma is a few minutes shorter than that (10–15 min) of protein-bound (nucleosomal) DNA (Grumaz et al. 2016; Elshimali et al. 2013). If this is also the case in pleural and peritoneal fluid, cfDNA from dead pathogens would be eliminated in a short time as there is no continuous release into the fluid, and the cfDNA from dead pathogens might not a severe problem for the ddPCR assay. A comprehensive research in biological characteristics of microbial cfDNA in pleural and peritoneal fluid would provide us with a better understanding to this problem.
In summary, the ddPCR assay proved to be a rapid and sensitive method for the detection of pleural and peritoneal infections. It is promising in clinical practice for optimizing treatment and improving outcomes of patients.
References
Amin M, Yousef Pour S, Navidifar T (2019) Detection of the major bacterial pathogens among children suffering from empyema in Ahvaz city, Iran. J Clin Lab Anal 33:e22855. https://doi.org/10.1002/jcla.22855
Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, Craig JA, Chudova DI, Devers PL, Jones KW, Oliver K, Rava RP, Sehnert AJ (2014) DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 370:799–808. https://doi.org/10.1056/NEJMoa1311037
Brogden KA, Guthmiller JM (eds) (2002) Polymicrobial diseases. ASM Press, Washington (DC). https://doi.org/10.1128/9781555817947
Click ES, Murithi W, Ouma GS, McCarthy K, Willby M, Musau S, Alexander H, Pevzner E, Posey J, Cain KP (2018) Detection of apparent cell-free M. tuberculosis DNA from plasma. Sci Rep 8:645. https://doi.org/10.1038/s41598-017-17683-6
Corcoran JP, Wrightson JM, Belcher E, DeCamp MM, Feller-Kopman D, Rahman NM (2015) Pleural infection: past, present, and future directions. Lancet Respir Med 3:563–577. https://doi.org/10.1016/S2213-2600(15)00185-X
Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A (2013) Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 10:472–484. https://doi.org/10.1038/nrclinonc.2013.110
Elshimali YI, Khaddour H, Sarkissyan M, Wu Y, Vadgama JV (2013) The clinical utilization of circulating cell free DNA (CCFDNA) in blood of cancer patients. Int J Mol Sci 14:18925–18958. https://doi.org/10.3390/ijms140918925
Franchetti L, Schumann D, Tamm M, Jahn K, Stolz D (2018) Efficacy of a multiplex bacterial PCR in identifying pathogens in pleural empyema. Eur Respir J 52(suppl 62):PA1966. https://doi.org/10.1183/13993003.congress-2018.PA1966
Grumaz S, Stevens P, Grumaz C, Decker SO, Weigand MA, Hofer S, Brenner T, von Haeseler A, Sohn K (2016) Next-generation sequencing diagnostics of bacteremia in septic patients. Genome Med 8:73. https://doi.org/10.1186/s13073-016-0326-8
Hardick J, Won H, Jeng K, Hsieh Y-H, Gaydos CA, Rothman RE, Yang S (2012) Identification of bacterial pathogens in ascitic fluids from patients with suspected spontaneous bacterial peritonitis by use of broad-range PCR (16S PCR) coupled with high-resolution melt analysis. J Clin Microbiol 50:2428–2432. https://doi.org/10.1128/jcm.00345-12
Hughes JA, Cabilan CJ, Williams J, Ray M, Coyer F (2018) The effectiveness of interventions to reduce peripheral blood culture contamination in acute care: a systematic review protocol. Syst Rev 7:216. https://doi.org/10.1186/s13643-018-0877-4
Jiménez D, Díaz G, García-Rull S, Vidal R, Sueiro A, Light RW (2006) Routine use of pleural fluid cultures. Are they indicated? Limited yield, minimal impact on treatment decisions. Respir Med 100:2048–2052. https://doi.org/10.1016/j.rmed.2006.02.008
Li X, Du W, Wang Y, Liu Z, Li K, Chen H, Liu R, Ma L, Zhang L, Dong Y, Che N, Gao M (2019) Rapid diagnosis of tuberculosis meningitis by detecting Mycobacterium tuberculosis cell-free DNA in cerebrospinal fluid. Am J Clin Pathol 153:126–130. https://doi.org/10.1093/ajcp/aqz135
Maskell NA, Batt S, Hedley EL, Davies CWH, Gillespie SH, Davies RJO (2006) The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med 174:817–823. https://doi.org/10.1164/rccm.200601-074OC
Menzies SM, Rahman NM, Wrightson JM, Davies HE, Shorten R, Gillespie SH, Davies CWH, Maskell NA, Jeffrey AA, Lee YCG, Davies RJO (2011) Blood culture bottle culture of pleural fluid in pleural infection. Thorax 66:658–662. https://doi.org/10.1136/thx.2010.157842
Picazo JJ, Contreras JR, Ríos E, Culebras E, Rodríguez-Avial I, Méndez C, Betriu C (2013) Rapid diagnosis of invasive pneumococcal disease in pediatric population. J Microbiol Methods 93:116–120. https://doi.org/10.1016/j.mimet.2013.03.001
She RC, Romney MG, Jang W, Walker T, Karichu JK, Richter SS (2018) Performance of the BacT/Alert Virtuo Microbial Detection System for the culture of sterile body fluids: prospective multicentre study. Clin Microbiol Infect 24:992–996. https://doi.org/10.1016/j.cmi.2017.12.011
Suo T, Liu X, Feng J, Guo M, Hu W, Guo D, Ullah H, Yang Y, Zhang Q, Wang X, Sajid M, Huang Z, Deng L, Chen T, Liu F, Xu K, Liu Y, Zhang Q, Liu Y, Xiong Y, Chen G, Lan K, Chen Y (2020) ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg Microbes Infect 9:1259–1268. https://doi.org/10.1080/22221751.2020.1772678
Ushio R, Yamamoto M, Nakashima K, Watanabe H, Nagai K, Shibata Y, Tashiro K, Tsukahara T, Nagakura H, Horita N, Sato T, Shinkai M, Kudo M, Ueda A, Kaneko T (2016) Digital PCR assay detection of circulating Mycobacterium tuberculosis DNA in pulmonary tuberculosis patient plasma. Tuberculosis (Edinb) 99:47–53. https://doi.org/10.1016/j.tube.2016.04.004
Vogelstein B, Kinzler KW (1999) Digital PCR. Proc Natl Acad Sci U S A 96:9236–9241. https://doi.org/10.1073/pnas.96.16.9236
Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N (2017) Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17:223–238. https://doi.org/10.1038/nrc.2017.7
Wang D, Hu Y, Li T, Rong H-M, Tong Z-H (2017) Diagnosis of Pneumocystis jirovecii pneumonia with serum cell-free DNA in non-HIV-infected immunocompromised patients. Oncotarget 8:71946–71953. https://doi.org/10.18632/oncotarget.18037
Weerakoon KG, McManus DP (2016) Cell-free DNA as a diagnostic tool for human parasitic infections. Trends Parasitol 32:378–391. https://doi.org/10.1016/j.pt.2016.01.006
White PL, Barnes RA, Springer J, Klingspor L, Cuenca-Estrella M, Morton CO, Lagrou K, Bretagne S, Melchers WJG, Mengoli C, Donnelly JP, Heinz WJ, Loeffler J (2015) Clinical performance of Aspergillus PCR for testing serum and plasma: a study by the European Aspergillus PCR Initiative. J Clin Microbiol 53:2832–2837. https://doi.org/10.1128/jcm.00905-15
Wouters Y, Dalloyaux D, Christenhusz A, Roelofs HMJ, Wertheim HF, Bleeker-Rovers CP, Te Morsche RH, Wanten GJA (2019) Droplet digital polymerase chain reaction for rapid broad-spectrum detection of bloodstream infections. Microb Biotechnol 13:657–668. https://doi.org/10.1111/1751-7915.13491
Wu YD, Li W, Wei Y, Gao HH, Shang SQ, Du LZ (2015) Rapid and sensitive identification of bacterial infection and bacteria gram types in pleural fluid of children. Glob Pediatr Health 2:2333794X15569302. https://doi.org/10.1177/2333794x15569302
Funding
This work was supported by research grants from Zhejiang Provincial Medical and Health Science and Technology project (no. 2017KY117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Medical Ethics Committee of the Hospital (No. 2019-KL-090-01) and was conducted in compliance with ethical, legal, and regulatory norms.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, F., Sun, S., Sun, X. et al. Rapid and sensitive identification of pleural and peritoneal infections by droplet digital PCR. Folia Microbiol 66, 213–219 (2021). https://doi.org/10.1007/s12223-020-00834-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-020-00834-0