Abstract
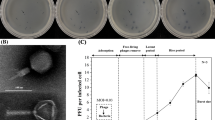
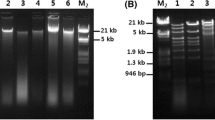
Among the various bacterial pathogens associated with the aquaculture environment, Vibrio parahaemolyticus the important one from shrimp and human health aspects. Though having been around for several decades, phage-based control of bacterial pathogens (phage therapy) has recently re-emerged as an attractive alternative due to the availability of modern phage characterization tools and the global emergence of antibiotic-resistant bacteria. In the present study, a total of 12 V. parahaemolyticus specific phages were isolated from 264 water samples collected from inland saline shrimp culture farms. During the host range analysis against standard/field isolates of V. parahaemolyticus and other bacterial species, lytic activity was observed against 2.3–45.5% of tested V. parahaemolyticus isolates. No lytic activity was observed against other bacterial species. For genomic characterization, high-quality phage nucleic acid with concentrations ranging from 7.66 to 220 ng/µl was isolated from 9 phages. After digestion treatments with DNase, RNase and S1 nuclease, the nature of phage nucleic acid was determined as ssDNA and dsDNA for 7 and 2 phages respectively. During transmission electron microscopy analysis of phage V5, it was found to have a filamentous shape making it a member of the family Inoviridae. During efficacy study of phage against V. parahaemolyticus in shrimp, 78.1% reduction in bacterial counts was observed within 1 h of phage application. These results indicate the potential of phage therapy for the control of V. parahaemoyticus in shrimp.




Similar content being viewed by others
References
FAO (2018) The state of world fisheries and aquaculture 2018—meeting the sustainable development goals. Rome
MPEDA (2018) India’s seafood export Cross US$ 7 billion for the first time. MPEDA, Kochi
Singh P, Tyagi A, Shanthanagouda AH, Naveen Kumar BT (2019) Status and scope of shrimp (Peneaus vannamei) farming in Punjab. In: Proceedings of World Brackishwater Aquaculture Conference (BRAQCON 2019), ICAR-Central Institute of Brackishwater Aquaculture (CIBA) & Society of Coastal Aquaculture and Fisheries (SCAFi), January 23–25, 2019, Chennai, 2019. Director, ICAR-CIBA, Chennai, pp 104
CSSRI (2010) Central Soil Salinity Research Institute. Karnal - 132001, India
Igbinosa EO (2016) Detection and antimicrobial resistance of vibrio isolates in aquaculture environments: implications for public health. Microb Drug Resist 22:238–245. https://doi.org/10.1089/mdr.2015.0169
Tyagi A, Saravanan V, Karunasagar I, Karunasagar I (2009) Detection of Vibrio parahaemolyticus in tropical shellfish by SYBR green real-time PCR and evaluation of three enrichment media. Int J Food Microbiol 129:124–130. https://doi.org/10.1016/j.ijfoodmicro.2008.11.006
Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV (2013) Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ 105:45–55. https://doi.org/10.3354/dao02621
Singh B, Tyagi A, Billekallu Thammegowda NK, Ansal MD (2018) Prevalence and antimicrobial resistance of vibrios of human health significance in inland saline aquaculture areas. Aquacult Res 49:2166–2174. https://doi.org/10.1111/are.13672
Yano Y, Hamano K, Satomi M, Tsutsui I, Ban M, Aue-umneoy D (2014) Prevalence and antimicrobial susceptibility of Vibrio species related to food safety isolated from shrimp cultured at inland ponds in Thailand. Food Control 38:30–36. https://doi.org/10.1016/j.foodcont.2013.09.019
Gordillo Altamirano FL, Barr JJ (2019) Phage therapy in the postantibiotic era. Clin Microbiol Rev. https://doi.org/10.1128/CMR.00066-18
Kim HJ, Ryu JO, Lee SY, Kim ES, Kim HY (2015) Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol 15:239. https://doi.org/10.1186/s12866-015-0577-3
Rai S, Tyagi A, Kumar BTN, Singh NK (2020) Optimization of plaque forming conditions for an Aeromonas hydrophila lytic bacteriophage. Int J Curr Microbiol App Sci 9:3764–3768. https://doi.org/10.20546/ijcmas.2020.906.445
Rai S, Tyagi A, Kumar BTN, Singh NK, Kaur S (2019) Isolation, genomic characterization and stability study of narrow-host range Aeromonas hydrophila lytic bacteriophage. J Exp Zool India 22:1075–1082
Rai S, Tyagi A, Kalia A, Kumar BTN, Garg P, Singh NK (2020) Characterization and genome sequencing of three Aeromonas hydrophila-specific phages, CF8, PS1, and PS2. Arch Virol. https://doi.org/10.1007/s00705-020-04644-0
Novik G, Ladutska A, Rakhuba D (2017) Bacteriophage taxonomy and classificaiton. In: Méndez-Vilas A (ed) Antimicrobial research: novel bioknowledge and educational programs. Formatex Research Center, Badajoz, pp 251–259
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Hyman P, Abedon ST (2010) Bacteriophage host range and bacterial resistance. Adv Appl Microbiol 70:217–248. https://doi.org/10.1016/S0065-2164(10)70007-1
Fischetti VA, Nelson D, Schuch R (2006) Reinventing phage therapy: are the parts greater than the sum? Nat Biotechnol 24:1508. https://doi.org/10.1038/nbt1206-1508
Silva YJ, Costa L, Pereira C, Mateus C, Cunha A, Calado R, Gomes NC, Pardo MA, Hernandez I, Almeida A (2014) Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 9:e114197. https://doi.org/10.1371/journal.pone.0114197
Cheepudom J, Lee CC, Cai B, Meng M (2015) Isolation, characterization, and complete genome analysis of P1312, a thermostable bacteriophage that infects Thermobifida fusca. Front Microbiol 6:959. https://doi.org/10.3389/fmicb.2015.00959
Székely AJ, Breitbart M (2016) Single-stranded DNA phages: from early molecular biology tools to recent revolutions in environmental microbiology. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnw027
Soffer N, Woolston J, Li M, Das C, Sulakvelidze A (2017) Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS ONE 12:e0175256. https://doi.org/10.1371/journal.pone.0175256
Jun JW, Kim HJ, Yun SK, Chai JY, Park SC (2014) Eating oysters without risk of vibriosis: application of a bacteriophage against Vibrio parahaemolyticus in oysters. Int J Food Microbiol 188:31–35. https://doi.org/10.1016/j.ijfoodmicro.2014.07.007
Zhang H, Yang Z, Zhou Y, Bao H, Wang R, Li T, Pang M, Sun L, Zhou X (2018) Application of a phage in decontaminating Vibrio parahaemolyticus in oysters. Int J Food Microbiol 275:24–31. https://doi.org/10.1016/j.ijfoodmicro.2018.03.027
Mai-Prochnow A, Hui JGK, Kjelleberg S, Rakonjac J, McDougald D, Rice SA (2015) Big things in small packages: the genetics of filamentous phage and effects on fitness of their host. FEMS Microbiol Rev 39:465–487. https://doi.org/10.1093/femsre/fuu007
Acknowledgements
The authors are grateful to the Dean, College of Fisheries, Guru Angad Dev Veterinary & Animal Sciences University, Ludhiana, India for facilities and support. This work was supported by Rashtriya Krishi Vikas Yojana (RKVY) Grant RKVY-11:I3 “Development of biotechnological intervention strategies to enhance the safety and shelf life of fishery products”.
Author information
Authors and Affiliations
Contributions
AT conceived the study, analyzed the data and drafted the manuscript. SD collected the samples and performed the experiments. AS contributed to phage efficacy studies. NBT and NKS contributed to phage genomic characterization. All authors contributed to manuscript correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no conflict of interest.
Ethical Approval
No special permission was required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dubey, S., Singh, A., Kumar, B.T.N. et al. Isolation and Characterization of Bacteriophages from Inland Saline Aquaculture Environments to Control Vibrio parahaemolyticus Contamination in Shrimp. Indian J Microbiol 61, 212–217 (2021). https://doi.org/10.1007/s12088-021-00934-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-021-00934-6