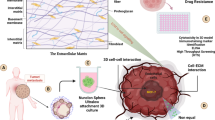
Abstract
While 3D cellular models are useful to study biological processes, gel-embedded organoids have large variability. This paper describes high-yield production of large (~1 mm diameter), scaffold-free, highly-spherical organoids in a one drop-one organoid format using MCF10A cells, a non-tumorigenic breast cell line. These organoids display a hollow lumen and secondary acini, and express mammary gland-specific and progenitor markers, resembling normal human breast acini. When subjected to treatment with TGF-β, the hypoxia-mimetic reagent CoCl2, or co-culture with mesenchymal stem/stromal cells (MSC), the organoids increase collagen I production and undergo large phenotypic and morphological changes of neoplastic progression, which were reproducible and quantifiable. Advantages of this scaffold-free, 3D breast organoid model include high consistency and reproducibility, ability to measure cellular collagen I production without noise from exogenous collagen, and capacity to subject the organoid to various stimuli from the microenvironment and exogenous treatments with precise timing without concern of matrix binding. Using this system, we generated organoids from primary metaplastic mammary carcinomas of MMTV-Cre;Ccn6fl/fl mice, which retained the high grade spindle cell morphology of the primary tumors. The platform is envisioned to be useful as a standardized 3D cellular model to study how microenvironmental factors influence breast tumorigenesis, and to potential therapeutics.






Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Abd El-Rehim DM et al (2004) Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 203:661–671
Aijian AP, Garrell RL (2015) Digital microfluidics for automated hanging drop cell spheroid culture. J Lab Autom 20:283–295
Anwar TE, Kleer CG (2013) Tissue-based identification of stem cells and epithelial-to- mesenchymal transition in breast cancer. Hum Pathol 44:1457–1464
Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ (1989) Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105:223–235
Bissell MJ, Hall HG, Parry G (1982) How does the extracellular matrix direct gene expression. J Theor Biol 99:31–68
Boecker W, Buerger H (2003) Evidence of progenitor cells of glandular and myoepithelial cell lineages in the human adult female breast epithelium: a new progenitor (adult stem) cell concept. Cell Prolif 36(Suppl 1):73–84
Celli JP et al (2014) An imaging-based platform for high-content, quantitative evaluation of therapeutic response in 3D tumour models. Sci Rep 4:1–10
Cerchiari AE et al (2015) A strategy for tissue self-organization that is robust to cellular heterogeneity and plasticity. Proc Natl Acad Sci 112:2287–2292
Chandler EM et al (2011) Stiffness of photocrosslinked RGD-alginate gels regulates adipose progenitor cell behavior. Biotechnol Bioeng 108:1683–1692
Chung W et al (2017) Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat Commun 8
Debnath J, Muthuswamy SK, Brugge JS (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30:256–268
Emerman J, Pitelka DR (1977) Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro 13:316–328
Fata JE et al (2007) The MAPKERK-1,2pathway integrates distinct and antagonistic signals from TGFα and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol 306:193–207
Gaiko-Shcherbak A et al (2015) The acinar cage: basement membranes determine molecule exchange and mechanical stability of human breast cell acini. PLoS One 10:1–20
Gonzalez ME et al (2017) Mesenchymal stem cell-induced DDR2 mediates stromal-breast Cancer interactions and metastasis growth. Cell Rep 18:1215–1228
Gusterson BA, Ross DT, Heath VJ, Stein T (2005) Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res 7:143–148
Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK (2003) Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 83:173–180
Klos KS et al (2014) Building bridges toward invasion: tumor promoter treatment induces a novel protein kinase C-dependent phenotype in MCF10A mammary cell acini. PLoS One 9:1–11
Lance A et al (2016) Increased extracellular matrix density decreases MCF10A breast cell acinus formation in 3D culture conditions. J Tissue Eng Regen Med 10:71–80
Lee GY, Kenny P a, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4:359–365
Lesher-Pérez SC et al (2017) Dispersible oxygen microsensors map oxygen gradients in three-dimensional cell cultures. Biomater. Sci. 5:2106–2113
Leung BM, Lesher-Perez SC, Matsuoka T, Moraes C, Takayama S (2015) Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci 3:336–344
Linnemann JR et al (2015) Quantification of regenerative potential in primary human mammary epithelial cells. Development 142:3239–3251
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Marina S, Bissell MJO (2017) A historical perspective of thinkin in three dimensions. J Cell Biol 216:1–10. https://doi.org/10.1083/jcb.201610056
Martin EE, Huang W, Anwar T, Arellano-Garcia C, Burman B, Guan J-L, Gonzalez ME, K CG (2017) MMTV-cre;Ccn6 knockout mice develop tumors recapitulating human metaplastic breast carcinomas. Oncogene 36:2275–2285
Mueller S, Millonig G, G W (2009) The GOX/CAT system: a novel enzymatic method to independently control hydrogen peroxide and hypoxia in cell culture. Adv Med Sci 54:121–135
Naba A, Hoersch S, Hynes RO (2012) Towards definition of an ECM parts list: an advance on GO categories. Matrix Biol 31:371–372
Naber HPH, Wiercinska E, ten Dijke P, van Laar T (2011) Spheroid assay to measure TGF-β-induced invasion. J Vis Exp:e3337. https://doi.org/10.3791/3337
Nakano T et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785
Pal A, Kleer CG (2014) Three dimensional cultures: A tool to study normal acinar architecture vs. malignant transformation of breast cells. J Vis Exp:e51311. https://doi.org/10.3791/51311
Pal A, Huang W, Toy KA, Kleer CG (2012) CCN6 knockdown disrupts acinar Organization of Breast Cells in three-dimensional cultures through up-regulation of type III TGF-β receptor. Neoplasia 14:1067–1074
Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ (1992) Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci 89:9064–9068
Qu Y et al (2015) Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells. PLoS One 10:1–16
Quadrato G et al (2017) Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545:48–53
Reid JA, Mollica PM, Bruno RD, Sachs PC (2018) Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res 20:1–13. https://doi.org/10.1186/s13058-018-1045-4
Rizwan A et al (2015) Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci Rep 5:1–6
Roerdink J, Meijster A (2000) The watershed transforms: definitions, algorithms and parallelization strategies. Fundam Inform 41(1-2):187–228. https://doi.org/10.3233/FI-2000-411207
Sachs N et al (2018) A living biobank of breast Cancer organoids captures disease heterogeneity. Cell 172:373–386.e10
Sato T et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772
Simian M et al (2001) The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 128:3117–3131
Thoma CR et al (2013) A high-throughput-compatible 3D microtissue co-culture system for phenotypic RNAi screening applications. J Biomol Screen 18:1330–1337
Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W (2014) 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 69–70:29–41
Tran DD, Corsa CAS, Biswas H, Aft RL, Longmore GD (2011) Temporal and spatial cooperation of Snail1 and Twist1 during epithelial-mesenchymal transition predicts for human breast Cancer recurrence. Mol Cancer Res 9:1644–1657
Tung Y-C et al (2011) High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136:473–478
Vaapil M et al (2012) Hypoxic conditions induce a Cancer-like phenotype in human breast epithelial cells. PLoS One 7:e46543
Venugopalan G et al (2014) Multicellular architecture of malignant breast epithelia influences mechanics. PLoS One 9:e101955
Vidi P, Bissell MJ, Lelièvre SA (2013) Epithelial Cell Culture Protocols 945:193–219
Vinci M et al (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 10:29
Wang M et al (2017) Role of tumor microenvironment in tumorigenesis. J Cancer 8:761–773
Weeber F, Ooft SN, Dijkstra KK, Voest EE (2017) Tumor organoids as a pre-clinical Cancer model for drug discovery. Cell Chem Biol 24:1092–1100
Wellings SR (1980) A hypothesis of the origin of human breast cancer from the terminal ductal lobular unit. Pathol Res Pract 166:515–535
Wetering M, De V et al (2015) Prospective derivation of a living organoid biobank of colorectal Cancer patients resource prospective derivation of a living organoid biobank of colorectal Cancer patients. Cell 161:933–945
Wu D, Yotnda P (2011) Induction and testing of hypoxia in cell culture. J Vis Exp:4–7. https://doi.org/10.3791/2899
Yin X et al (2016) Cell stem cell engineering stem cell organoids. Stem Cell 18:25–38
Zanoni M et al (2016) 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci Rep 6:1–11
Zhang J et al (2015) TGF- b – induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci Signal 7:1–12
Zhou Y et al (2013) Multiparameter analyses of three-dimensionally cultured tumor spheroids based on respiratory activity and comprehensive gene expression profiles. Anal Biochem 439:187–193
Zhu J, Xiong G, Trinkle C (2014) Xu, R. integrated extracellular matrix signaling in mammary gland development and breast cancer progression. Histol Histopathol 29:1083–1092
Ziółkowska K et al (2012) Development of a three-dimensional microfluidic system for long-term tumor spheroid culture. Sensors Actuators B Chem 173:908–913
Acknowledgements
We thank Tina Fields (Research Histology and IHC Laboratory, Rogel Cancer Center, University of Michigan) and Dafydd Thomas (Department of Pathology, Michigan Medicine) for their assistance with sectioning, immunohistochemistry, and immunostaining procedures and Dr. Brendan Leung for early studies. The study was supported by R01 grants to Dr. Celina Kleer (R01 CA107469, R01 CA125577, and the Karlene Kulp Fund Judy & Ken Robinson Fund), Dr. Shuichi Takayama (R01 CA196018), and the University of Michigan Rogel Cancer Center support grant P30CA046592. The authors would also like to thank Dr. Alexey Nesvizhskii for continued support by the Proteome Informatics of Cancer Training Program (PICTP) (NIH 5T32CA140044-08).
Author information
Authors and Affiliations
Contributions
S.D., C.K., and S.T. were involved in the study conception, design, analyses and interpretation. S.D. performed data acquisition and analysis, and B.B. and M.M. helped with completion of experiments and interpretation. S.D. prepared the manuscript and figures, C.K. and S.T. helped with the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing financial interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Figure 1
Spheroid optimization assay at varying Matrigel concentrations (0%, 1%, 1.5% and 2% v/v) from time = 0 to 72 h (N = 25 per subgroup) indicated by A) representative brightfield images, B) average spheroid diameter, C) percentage of droplets containing multi-spheres per droplet, called “loose cell aggregates”, and D) percentage of droplets that form one sphere per droplet. Scalebar = 100 μm. (PPTX 4365 kb)
Supplemental Figure 2
Comparison of stem cell vs differentiation marker expression for western blot analysis of MCF10A cells cultured in monolayer and 3D at days 4 and 8, where “MG−” identifies MCF10A spheroids seeded without Matrigel. MDA-MB-231 cells cultured in 2D were shown as a control. Antibodies used: E-cadherin, Vimentin, ALDH1, CD44, CD49f, EpCam, and β-actin. Values above E-cadherin blot shows densitometric analysis of relative concentrations. (PPTX 72 kb)
Supplemental Figure 3
Summary of organoids developed in hanging drop with A) MCF10A vs MDA-MB-231 organoids at days 4, 8, and 16 with a comparison of monolayer phase contrast images, H&E staining, and confocal imaging with DAPI, CK5/6, and CK18 status (expression results merged), B) comparison of H&E and collagen I staining from MCF10A, TGFβ1, CoCl2, and co-culture organoids at days 8, 12, and 16. Scale bar =200 μm. (PPTX 16956 kb)
Rights and permissions
About this article
Cite this article
Djomehri, S.I., Burman, B., Gonzalez, M.E. et al. A reproducible scaffold-free 3D organoid model to study neoplastic progression in breast cancer. J. Cell Commun. Signal. 13, 129–143 (2019). https://doi.org/10.1007/s12079-018-0498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-018-0498-7