Abstract
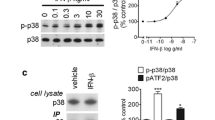
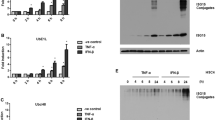
Interferon gamma (IFN-ɣ) is a pleiotropic cytokine which plays dual contrasting roles in cancer. Although IFN-ɣ has been clinically used to treat various malignancies, it was recently shown to have protumorigenic activities. Reactive oxygen species (ROS) are overproduced in cancer cells, mainly due to NADPH oxidase activity, which results into several changes in signaling pathways. In this study, we examined IFN-ɣ effect on the phosphorylation levels of key signaling proteins, through ROS production, in the human breast cancer cell line MCF-7. After treatment by IFN-ɣ, results showed a significant increase in the phosphorylation of STAT1, Src, raf, AKT, ERK1/2 and p38 signaling molecules, in a time specific manner. Src and Raf were found to be involved in early stages of IFN-ɣ signaling since their phosphorylation increased very rapidly. Selective inhibition of Src-family kinases resulted in an immediate significant decrease in the phosphorylation status of Raf and ERK1/2, but not p38 and AKT. On the other hand, IFN-ɣ resulted in ROS generation, through H2O2 production, whereas pre-treatment with the ROS inhibitor NAC caused ROS inhibition and a significant decrease in the phosphorylation levels of AKT, ERK1/2, p38 and STAT1. Moreover, pretreatment with a selective NOX1 inhibitor resulted in a significant decrease of AKT phosphorylation. Finally, no direct relationship was found between ROS production and calcium mobilization. In summary, IFN-ɣ signaling in MCF-7 cell line is ROS-dependent and follows the Src/Raf/ERK pathway whereas its signaling through the AKT pathway is highly dependent on NOX1.








Similar content being viewed by others
References
Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M (2015) IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer 112(9):1501–9
Babior BM, Lambeth JD, Nauseef W (2002) The neutrophil NADPH oxidase. Arch Biochem Biophys 397:342–344
Buechter DD (1988) Free radicals and oxygen toxicity. Pharm Res 5:253–60
Cadwallader KA (2002) Regulation of phosphatidylinositol 3-kinase activity and phosphatidylinositol 3,4,5-trisphosphate accumulation by neutrophil priming agents. J Immunol 169:3336–3344
Cassatella MA (1990) Molecular basis of interferon-γ and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components J Biol Chem 265(20241–20246)
Droge W (2002) Free Radicals in the physiological control of cell function. Physiol Rev 82:47–95
Ealick SE, Cook WJ, Vijay-Kumar S, Carson M, Nagabhushan TL, Trotta PP, Bugg CE (1991) Three-dimensional structure of recombinant human interferon-gamma. Science 252:698–702
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194:7–15
Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P (2016) Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 167(2):397–404.e9. doi:10.1016/j.cell.2016.08.069
Haan C, Kreis S, Margue C, Behrmann I (2006) Jaks and cytokine receptors an intimate relationship. Biochem Pharmacol 72:1538–46
Halliwell B (1999) Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res 443:37–52
Hu X, Ivashkiv LB (2009) Cross-regulation of signaling pathways by interferon gamma: implications for immune responses and autoimmune diseases. Immunity 31:539–50
Kearney SJ, Delgado C, Eshleman EM, Hill KK, O’Connor BP, Lenz LL (2013) Type I IFNs downregulate myeloid cell IFN-γ receptor by inducing recruitment of an early growth response 3/NGFI-A binding protein 1 complex that silences IFN-ɣ R1 transcription. J Immunol 191(6):3384–92
Krämer OH, Knauer SK, Greiner G, Jandt E, Reichardt S, Gührs KH, Stauber RH, Böhmer FD, Heinzel T (2009) A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev 23:223–35
Kumatori A, Yang D, Suzuki S, Nakamura M (2002) Cooperation of STAT-1 and IRF-1 in interferon-gamma-induced transcription of the gp91 (phox) gene. J Biol Chem 277:9103–9111
Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K (2006) Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol 290:C433–C443
Lambeth JD (2004) NOX enzymes and the biology of reactive oxygen. Nature Reviews 4:181–189
Lollini PL, Bosco MC, Cavallo F, De Giovanni C, Giovarelli M, Landuzzi L, Musiani P, Modesti A, Nicoletti G, Palmieri G (1993) Inhibition of tumor growth and enhancement of metastasis after transfection of the gamma-interferon gene. Int J Cancer 55:320–9
Min W, Pober J, Johnson D (1996) Kinetically coordinated induction of TAP1 and HLA class I by IFN-γ: the rapid induction of TAP1 by IFN-γ is mediated by STAT1. J Immunol 156:3174–3183
Pervaiz S (2004) MV Clement.: Tumor intracellular redox status and drug resistance - serendipity or a causal relationship? Curr Pharm Des 10(1969–1977)
Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signaling. Nat Rev Immunol 5:375–86
Resnitzky D, Tiefenbrun T, Berissi H, Kimchi A (1992) Interferons and interleukin 6 suppress phosphorylation of the retinoblastoma protein in growth-sensitive hematopoietic cells. Proc Natl Acad Sci USA 89:402–406
Sesti F, Tsitsilonis OE, Kotsinas A, Trougakos IP (2012) Oxidative stress-mediated biomolecular damage and inflammation in tumorigenesis. In Vivo 26:395–402
Shi LZ, Fu T, Guan B, Chen J, Blando JM, Allison JP, Xiong L, Subudhi SK, Gao J, Sharma P (2016) Interdependent IL-7 and IFN-γ signalling in T-cell controls tumour eradication by combined α-CTLA-4 + α-PD-1 therapy. Nat Commun 7:12335. doi:10.1038/ncomms12335
Subramaniam P, Johnson H (1997) A role for the cyclin-dependent kinase inhibitor p21 in the G1 cell cycle arrest mediated by type I interferons. J Interferon Cytokine Res 17:11–15
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299
Toyokuni S, Okamoto K, Yodoi J, Hiai H (1995) Persistent oxidative stress in cancer. FEBS Lett 358:1–3
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374
Zhong M, Henriksen MA, Takeuchi K, Schaefer O, Liu B, Ten Hoeve J, Ren Z, Mao X, Chen X, Shuai K, Darnell JE Jr (2005) Implications of an antiparallel dimeric structure of non-phosphorylated STAT1 for the activation-inactivation cycle. Proc Natl Acad Sci U S A 102:3966–71
Acknowledgements
This work was supported by grants from Lebanese National Council for Scientific Research (KZ) and Lebanese University grants (KZ, NEZ).
Author contributions statement
HB, NEZ performed experiments. AZ, NK & BB prepared Figures. KZ & NEZ designed the study, analyzed data and wrote the manuscript. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict-of-interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zibara, K., Zeidan, A., Bjeije, H. et al. ROS mediates interferon gamma induced phosphorylation of Src, through the Raf/ERK pathway, in MCF-7 human breast cancer cell line. J. Cell Commun. Signal. 11, 57–67 (2017). https://doi.org/10.1007/s12079-016-0362-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-016-0362-6