Abstract
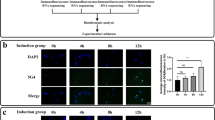
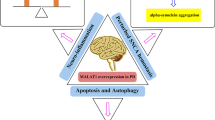
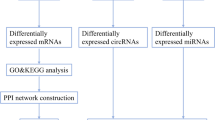
In clinical practice, the underlying pathogenesis of Parkinson’s disease (PD) remains unknown. Circular RNAs (circRNAs) have good biological properties and can be used as biological marker. Rehabilitation as a third treatment alongside drug and surgery has been shown to be clinically effective, but biomarkers of rehabilitation efficiency at genetic level is still lacking. In this study, we identified differentially expressed circRNAs in peripheral blood exosomes between PD patients and health controls (HCs) and determined whether these circRNAs changed after rehabilitation, to explore the competing RNA networks and epigenetic mechanisms affected. We found that there were 558 upregulated and 609 downregulated circRNAs in PD patients compared to HCs, 3398 upregulated and 479 downregulated circRNAs in PD patients after rehabilitation compared to them before rehabilitation, along with 3721 upregulated and 635 downregulated circRNAs in PD patients after rehabilitation compared to HCs. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that differentially expressed circRNAs may affect the stability of the cellular actin backbone and synaptic structure by influencing the aggregation of α-synuclein (a-syn). We selected two circRNAs overexpressed in PD patients for validation (hsa_circ_0001535 and hsa_circ_0000437); the results revealed that their expression levels were all reduced to varying degrees (p < 0.05) after rehabilitation. After network analysis, we believe that hsa_circ_0001535 may be related to the aggregation of a-syn, while hsa_circ_0000437 may act on hsa-let-7b-5p or hsa-let-7c-5p through sponge effect to cause inflammatory response. Our findings suggest that rehabilitation can mitigate the pathological process of PD by epigenetic means.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available in the online repository, https://ngdc.cncb.ac. cn/gsa-human/, HRA002383.
References
Farrer MJ (2006) Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 7(4):306–318. https://doi.org/10.1038/nrg1831
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Tolosa E, Wenning G, Poewe W (2006) The diagnosis of Parkinson’s disease. Lancet Neurol 5(1):75–86. https://doi.org/10.1016/s1474-4422(05)70285-4
Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Mov Disord 26(6):1049–1055. https://doi.org/10.1002/mds.23732
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. https://doi.org/10.1016/s1474-4422(16)30230-7
Liu H, Koros C, Strohäker T, Schulte C, Bozi M, Varvaresos S, Ibáñez de Opakua A, Simitsi AM et al (2021) A novel SNCA A30G mutation causes familial Parkinson’s disease. Mov Disord 36(7):1624–1633. https://doi.org/10.1002/mds.28534
Burré J, Sharma M, Südhof TC (2018) Cell biology and pathophysiology of α-synuclein. Cold Spring Harb Perspect Med 8(3). https://doi.org/10.1101/cshperspect.a024091
Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2009) Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol 41(10):2015–2024. https://doi.org/10.1016/j.biocel.2009.05.008
Garcia-Esparcia P, Hernández-Ortega K, Koneti A, Gil L, Delgado-Morales R, Castaño E, Carmona M, Ferrer I (2015) Altered machinery of protein synthesis is region- and stage-dependent and is associated with α-synuclein oligomers in Parkinson’s disease. Acta Neuropathol Commun 3:76. https://doi.org/10.1186/s40478-015-0257-4
Peschansky VJ, Wahlestedt C (2014) Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 9(1):3–12. https://doi.org/10.4161/epi.27473
Migliore L, Coppedè F (2022) Gene-environment interactions in Alzheimer disease: the emerging role of epigenetics. Nat Rev Neurol 18(11):643–660. https://doi.org/10.1038/s41582-022-00714-w
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J (2019) The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 20(11):675–691. https://doi.org/10.1038/s41576-019-0158-7
Kelly S, Greenman C, Cook PR, Papantonis A (2015) Exon skipping is correlated with exon circularization. J Mol Biol 427(15):2414–2417. https://doi.org/10.1016/j.jmb.2015.02.018
Kramer MC, Liang D, Tatomer DC, Gold B, March ZM, Cherry S, Wilusz JE (2015) Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes Dev 29(20):2168–2182. https://doi.org/10.1101/gad.270421.115
Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342. https://doi.org/10.1038/nature10098
Ravanidis S, Bougea A, Karampatsi D, Papagiannakis N, Maniati M, Stefanis L, Doxakis E (2021) Differentially expressed circular RNAs in Peripheral blood mononuclear cells of patients with Parkinson’s disease. Mov Disord 36(5):1170–1179. https://doi.org/10.1002/mds.28467
Hanan M, Simchovitz A, Yayon N, Vaknine S, Cohen-Fultheim R, Karmon M, Madrer N, Rohrlich TM et al (2020) A Parkinson’s disease CircRNAs Resource reveals a link between circSLC8A1 and oxidative stress. EMBO Mol Med 12(9):e11942. https://doi.org/10.15252/emmm.201911942
Miller DB, O’Callaghan JP (2015) Biomarkers of Parkinson’s disease: present and future. Metabolism 64(3 Suppl 1):S40-46. https://doi.org/10.1016/j.metabol.2014.10.030
Fisher BE, Li Q, Nacca A, Salem GJ, Song J, Yip J, Hui JS, Jakowec MW et al (2013) Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. NeuroReport 24(10):509–514. https://doi.org/10.1097/WNR.0b013e328361dc13
Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M, Practice Recommendations Development G (2007) Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord 22(4):451–460; quiz 600. https://doi.org/10.1002/mds.21244
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601. https://doi.org/10.1002/mds.26424
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG et al (2004) Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19(9):1020–1028. https://doi.org/10.1002/mds.20213
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Mathias S, Nayak US, Isaacs B (1986) Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 67(6):387–389
Combs SA, Diehl MD, Filip J, Long E (2014) Short-distance walking speed tests in people with Parkinson disease: reliability, responsiveness, and validity. Gait Posture 39(2):784–788. https://doi.org/10.1016/j.gaitpost.2013.10.019
Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB (1985) The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132(8):919–923
Duncan RP, Leddy AL, Earhart GM (2011) Five times sit-to-stand test performance in Parkinson’s disease. Arch Phys Med Rehabil 92(9):1431–1436. https://doi.org/10.1016/j.apmr.2011.04.008
Potter K, Brandfass K (2015) The Mini-Balance Evaluation Systems Test (Mini-BESTest). J Physiother 61(4):225. https://doi.org/10.1016/j.jphys.2015.04.002
Thompson E (2015) Hamilton Rating Scale for Anxiety (HAM-A). Occup Med (Lond) 65(7):601. https://doi.org/10.1093/occmed/kqv054
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23(1):56–62. https://doi.org/10.1136/jnnp.23.1.56
Wang Y, Liu Y, Jin Z, Liu C, Yu X, Chen K, Meng D, Liu A et al (2022) Association between mitochondrial function and rehabilitation of Parkinson’s disease: revealed by exosomal mRNA and lncRNA expression profiles. Front Aging Neurosci 14:909622. https://doi.org/10.3389/fnagi.2022.909622
Chen KK, Jin ZH, Gao L, Qi L, Zhen QX, Liu C, Wang P, Liu YH et al (2021) Efficacy of short-term multidisciplinary intensive rehabilitation in patients with different Parkinson’s disease motor subtypes: a prospective pilot study with 3-month follow-up. Neural Regen Res 16(7):1336–1343. https://doi.org/10.4103/1673-5374.301029
Mandolesi G, Rizzo FR, Balletta S, Stampanoni Bassi M, Gilio L, Guadalupi L, Nencini M, Moscatelli A et al (2021) The microRNA let-7b-5p is negatively associated with inflammation and disease severity in multiple sclerosis. Cells 10(2). https://doi.org/10.3390/cells10020330
Lv J, Zeng Y, Qian Y, Dong J, Zhang Z, Zhang J (2018) MicroRNA let-7c-5p improves neurological outcomes in a murine model of traumatic brain injury by suppressing neuroinflammation and regulating microglial activation. Brain Res 1685:91–104. https://doi.org/10.1016/j.brainres.2018.01.032
Verstraeten A, Theuns J, Van Broeckhoven C (2015) Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet 31(3):140–149. https://doi.org/10.1016/j.tig.2015.01.004
Gunnarsson LG, Bodin L (2019) Occupational exposures and neurodegenerative diseases-a systematic literature review and meta-analyses. Int J Environ Res Public Health 16(3). https://doi.org/10.3390/ijerph16030337
Więckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U (2021) Western diet as a trigger of Alzheimer’s disease: from metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev 70:101397. https://doi.org/10.1016/j.arr.2021.101397
Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA et al (2015) The epigenetics of aging and neurodegeneration. Prog Neurobiol 131:21–64. https://doi.org/10.1016/j.pneurobio.2015.05.002
van Heesbeen HJ, Smidt MP (2019) Entanglement of genetics and epigenetics in Parkinson’s disease. Front Neurosci 13:277. https://doi.org/10.3389/fnins.2019.00277
Jiang C, Hopfner F, Katsikoudi A, Hein R, Catli C, Evetts S, Huang Y, Wang H et al (2020) Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry 91(7):720–729. https://doi.org/10.1136/jnnp-2019-322588
Pavlou MAS, Pinho R, Paiva I, Outeiro TF (2017) The yin and yang of α-synuclein-associated epigenetics in Parkinson’s disease. Brain 140(4):878–886. https://doi.org/10.1093/brain/aww227
Thomson DW, Dinger ME (2016) Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17(5):272–283. https://doi.org/10.1038/nrg.2016.20
Starke S, Jost I, Rossbach O, Schneider T, Schreiner S, Hung LH, Bindereif A (2015) Exon circularization requires canonical splice signals. Cell Rep 10(1):103–111. https://doi.org/10.1016/j.celrep.2014.12.002
Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin QF, Wei J, Yao RW et al (2017) Coordinated circRNA Biogenesis and function with NF90/NF110 in Viral Infection. Mol Cell 67(2):214-227.e217. https://doi.org/10.1016/j.molcel.2017.05.023
Kristensen LS, Hansen TB, Venø MT, Kjems J (2018) Circular RNAs in cancer: opportunities and challenges in the field. Oncogene 37(5):555–565. https://doi.org/10.1038/onc.2017.361
Svitkina T (2018) The actin cytoskeleton and actin-based motility. Cold Spring Harb Perspect Biol 10(1). https://doi.org/10.1101/cshperspect.a018267
Carlier MF (1998) Control of actin dynamics. Curr Opin Cell Biol 10(1):45–51. https://doi.org/10.1016/s0955-0674(98)80085-9
Eira J, Silva CS, Sousa MM, Liz MA (2016) The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog Neurobiol 141:61–82. https://doi.org/10.1016/j.pneurobio.2016.04.007
Carnwath T, Mohammed R, Tsiang D (2018) The direct and indirect effects of α-synuclein on microtubule stability in the pathogenesis of Parkinson’s disease. Neuropsychiatr Dis Treat 14:1685–1695. https://doi.org/10.2147/ndt.S166322
Oliveira da Silva MI, Liz MA (2020) Linking alpha-synuclein to the actin cytoskeleton: consequences to neuronal function. Front Cell Dev Biol 8:787. https://doi.org/10.3389/fcell.2020.00787
Bellani S, Mescola A, Ronzitti G, Tsushima H, Tilve S, Canale C, Valtorta F, Chieregatti E (2014) GRP78 clustering at the cell surface of neurons transduces the action of exogenous alpha-synuclein. Cell Death Differ 21(12):1971–1983. https://doi.org/10.1038/cdd.2014.111
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M et al (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44(4):601–607. https://doi.org/10.1016/j.neuron.2004.11.005
Islam MS, Moore DJ (2017) Mechanisms of LRRK2-dependent neurodegeneration: role of enzymatic activity and protein aggregation. Biochem Soc Trans 45(1):163–172. https://doi.org/10.1042/bst20160264
Biosa A, Trancikova A, Civiero L, Glauser L, Bubacco L, Greggio E, Moore DJ (2013) GTPase activity regulates kinase activity and cellular phenotypes of Parkinson’s disease-associated LRRK2. Hum Mol Genet 22(6):1140–1156. https://doi.org/10.1093/hmg/dds522
Liu X, Gao Y, Lin X, Li L, Han X, Liu J (2016) The coronin family and human disease. Curr Protein Pept Sci 17(6):603–611. https://doi.org/10.2174/1389203717666151201192011
Lai D, Alipanahi B, Fontanillas P, Schwantes-An TH, Aasly J, Alcalay RN, Beecham GW, Berg D et al (2021) Genomewide association studies of LRRK2 modifiers of Parkinson’s disease. Ann Neurol 90(1):76–88. https://doi.org/10.1002/ana.26094
Xie Y, Hang X, Xu W, Gu J, Zhang Y, Wang J, Zhang X, Cao X et al (2021) CircFAM13B promotes the proliferation of hepatocellular carcinoma by sponging miR-212, upregulating E2F5 expression and activating the P53 pathway. Cancer Cell Int 21(1):410. https://doi.org/10.1186/s12935-021-02120-6
Luo Q, Sun W, Wang YF, Li J, Li DW (2022) Association of p53 with neurodegeneration in Parkinson’s disease. Parkinsons Dis 2022:6600944. https://doi.org/10.1155/2022/6600944
García S, Amor-Gutiérrez O, Palomares-Albarrán M, Toyos-Rodríguez C, Cuetos F, Martínez C, Costa-García A, Fernández-Sánchez MT et al (2021) Unfolded p53 as a marker of oxidative stress in mild cognitive impairment, Alzheimer’s and Parkinson’s disease. Curr Alzheimer Res 18(9):695–700. https://doi.org/10.2174/1567205018666211117101216
Dai CQ, Luo TT, Luo SC, Wang JQ, Wang SM, Bai YH, Yang YL, Wang YY (2016) p53 and mitochondrial dysfunction: novel insight of neurodegenerative diseases. J Bioenerg Biomembr 48(4):337–347. https://doi.org/10.1007/s10863-016-9669-5
Duplan E, Giordano C, Checler F, Alves da Costa C (2016) Direct α-synuclein promoter transactivation by the tumor suppressor p53. Mol Neurodegener 11:13. https://doi.org/10.1186/s13024-016-0079-2
Wu F, Sun G, Zheng W, Tang W, Cheng Y, Wu L, Li X, Tao J et al (2021) circCORO1C promotes the proliferation and metastasis of hepatocellular carcinoma by enhancing the expression of PD-L1 through NF-kappaB pathway. J Clin Lab Anal 35(12):e24003. https://doi.org/10.1002/jcla.24003
Li Y, Xia Y, Yin S, Wan F, Hu J, Kou L, Sun Y, Wu J et al (2021) Targeting microglial alpha-synuclein/TLRs/NF-kappaB/NLRP3 inflammasome axis in Parkinson’s disease. Front Immunol 12:719807. https://doi.org/10.3389/fimmu.2021.719807
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V (2015) Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem J 471(3):323–333. https://doi.org/10.1042/bj20150617
Chen G, Xie D, Zhang P, Zhou H (2022) Circular RNA hsa_circ_0000437 may be used as a new indicator for the diagnosis and prognosis of hepatocellular carcinoma. Bioengineered 13(6):14118–14124. https://doi.org/10.1080/21655979.2022.2081458
Li F, Cai Y, Deng S, Yang L, Liu N, Chang X, Jing L, Zhou Y et al (2021) A peptide CORO1C-47aa encoded by the circular noncoding RNA circ-0000437 functions as a negative regulator in endometrium tumor angiogenesis. J Biol Chem 297(5):101182. https://doi.org/10.1016/j.jbc.2021.101182
Schratt G (2009) microRNAs at the synapse. Nat Rev Neurosci 10(12):842–849. https://doi.org/10.1038/nrn2763
Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS (2018) Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b–5p and Let-7b-5p Regulate Macrophage Cytokine Production. J Clin Endocrinol Metab 103(1):64–74. https://doi.org/10.1210/jc.2017-01199
Wu Y, Zhang Y, Zheng X, Dai F, Lu Y, Dai L, Niu M, Guo H et al (2020) Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Mol Cancer 19(1):99. https://doi.org/10.1186/s12943-020-01215-4
Redila VA, Christie BR (2006) Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience 137(4):1299–1307. https://doi.org/10.1016/j.neuroscience.2005.10.050
Johansson ME, Cameron IGM, Van der Kolk NM, de Vries NM, Klimars E, Toni I, Bloem BR, Helmich RC (2022) Aerobic exercise alters brain function and structure in Parkinson’s disease: a randomized controlled trial. Ann Neurol 91(2):203–216. https://doi.org/10.1002/ana.26291
Acknowledgements
The authors wish to thank Pro.BF for her constant guidance through all the stages of the writing of this manuscript.
Funding
This work was supported by the Science and Technology Development Fund of Beijing Rehabilitation Hospital, Capital Medical University (2019–023 to YL, 2020–069, 2021–011 to BF, and 2020R-001 to YW).
Author information
Authors and Affiliations
Contributions
JX and BF contributed to the study conception and design. Material preparation and data collection were performed by YL, ZJ, CL, XY, KC, and DM. Statistical analysis was performed by YD and YW. The first draft of the manuscript was written by YD, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The studies involving human participants. Approval was granted by the Ethics Committee of Beijing Rehabilitation Hospital of Capital Medical University (2020bkky010).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, Y., Wang, Y., Liu, Y. et al. Circular RNAs in Parkinson’s Disease: Reliable Biological Markers and Targets for Rehabilitation. Mol Neurobiol 60, 3261–3276 (2023). https://doi.org/10.1007/s12035-023-03268-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03268-0