Abstract
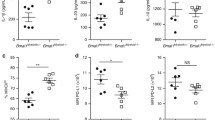
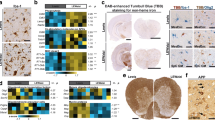
Both immune and neurodegenerative mechanisms underlie multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). MS/EAE are triggered by encephalitogenic immune cells, including Th1 and Th17 cells, whereas T regulatory (Treg) cells are involved in inflammation resolution. Pro-inflammatory macrophages/microglia also play a deleterious role in the disease. Seasonal variations in MS relapses, active lesions, and pro- and anti-inflammatory cytokine levels have been described in MS patients and have been related with both perinatal and adult exposure to sunlight and other environmental factors. However, some data in EAE mice suggest that these variations might be, at least partially, endogenously determined. Thus, our objective was to study the effect of the season of birth and disease induction on the course of EAE, and immune cell infiltration in the central nervous system (CNS) in myelin oligodendrocyte glycoprotein (MOG35–55)-induced EAE in 8 weeks old, female C57BL/6N mice maintained under constant, controlled conditions. EAE severity as well as pathogenic (Th1, Th17, macrophages/microglia) and protective (Treg) subsets was found to vary according to the season of birth or of EAE induction. Summer-born or summer-immunized animals developed a milder disease, which coincided with variations in numbers of T effector/regulatory subsets, and significantly low numbers of macrophages/microglia. These results suggest that endogenous rhythms in immune responses might cause seasonal variations in EAE severity, and, maybe, in the course of MS, and that they might be related to macrophages/microglia.


Similar content being viewed by others
Abbreviations
- BBB:
-
Blood-brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- EAE:
-
Experimental autoimmune encephalomyelitis
- IFN:
-
Interferon
- IL:
-
Interleukin
- MS:
-
Multiple sclerosis
- MRI:
-
Magnetic resonance imaging
- NK:
-
Natural killer cells
- Th:
-
T helper cell
- TNF:
-
Tumoral necrosis factor
- Treg:
-
T regulatory cells
References
Atlas of MS 2013: Mapping multiple sclerosis around the world (2013) Avaliable at: http://www.msif.org/about-us/advocacy/atlas/. http://www.msif.org/about-us/advocacy/atlas/
Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J, Group MS, Platform EMS (2017) New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 23(8):1123–1136. https://doi.org/10.1177/1352458517694432
Kuhlmann T, Ludwin S, Prat A, Antel J, Bruck W, Lassmann H (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 133(1):13–24. https://doi.org/10.1007/s00401-016-1653-y
Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S et al (2002) Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8(5):500–508. https://doi.org/10.1038/nm0502-500
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172(1):146–155. https://doi.org/10.2353/ajpath.2008.070690
Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J et al (2000) Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6(10):1167–1175. https://doi.org/10.1038/80516
Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E (2009) Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 132(Pt 12):3329–3341. https://doi.org/10.1093/brain/awp289
Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK (2004) Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 200(1):79–87. https://doi.org/10.1084/jem.20031819
Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y (2006) IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 177(1):566–573. https://doi.org/10.4049/jimmunol.177.1.566
McGeachy MJ, Stephens LA, Anderton SM (2005) Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol 175(5):3025–3032
Fritzsching B, Haas J, Konig F, Kunz P, Fritzsching E, Poschl J, Krammer PH, Bruck W et al (2011) Intracerebral human regulatory T cells: analysis of CD4+ CD25+ FOXP3+ T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PLoS One 6(3):e17988. https://doi.org/10.1371/journal.pone.0017988
Singh S, Metz I, Amor S, van der Valk P, Stadelmann C, Bruck W (2013) Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol 125(4):595–608. https://doi.org/10.1007/s00401-013-1082-0
Almolda B, Costa M, Montoya M, Gonzalez B, Castellano B (2009) CD4 microglial expression correlates with spontaneous clinical improvement in the acute Lewis rat EAE model. J Neuroimmunol 209(1–2):65–80. https://doi.org/10.1016/j.jneuroim.2009.01.026
Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM (2011) Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14(9):1142–1149. https://doi.org/10.1038/nn.2887
Bhasin M, Wu M, Tsirka SE (2007) Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis. BMC Immunol 8:10. https://doi.org/10.1186/1471-2172-8-10
Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, Guo M, Xie Y et al (2013) Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PLoS One 8(2):e54841. https://doi.org/10.1371/journal.pone.0054841
Harding K, Tilling K, MacIver C, Willis M, Joseph F, Ingram G, Hirst C, Wardle M et al (2017) Seasonal variation in multiple sclerosis relapse. J Neurol 264(6):1059–1067. https://doi.org/10.1007/s00415-017-8485-0
Spelman T, Gray O, Trojano M, Petersen T, Izquierdo G, Lugaresi A, Hupperts R, Bergamaschi R et al (2014) Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol 76(6):880–890. https://doi.org/10.1002/ana.24287
Hartl C, Obermeier V, Gerdes LA, Brugel M, von Kries R, Kumpfel T (2017) Seasonal variations of 25-OH vitamin D serum levels are associated with clinical disease activity in multiple sclerosis patients. J Neurol Sci 375:160–164. https://doi.org/10.1016/j.jns.2017.01.059
Auer DP, Schumann EM, Kumpfel T, Gossl C, Trenkwalder C (2000) Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 47(2):276–277. https://doi.org/10.1002/1531-8249(200002)47:2<276::AID-ANA28>3.0.CO;2-1
Meier DS, Balashov KE, Healy B, Weiner HL, Guttmann CR (2010) Seasonal prevalence of MS disease activity. Neurology 75(9):799–806. https://doi.org/10.1212/WNL.0b013e3181f0734c
Damasceno A, Moraes AS, Farias A, Damasceno BP, dos Santos LM, Cendes F (2016) A spring to summer shift of pro-inflammatory cytokine production in multiple sclerosis patients. J Neurol Sci 360:37–40. https://doi.org/10.1016/j.jns.2015.11.022
Stewart N, Taylor B, Ponsonby AL, Pittas F, van der Mei I, Woods G, Walters H (2007) The effect of season on cytokine expression in multiple sclerosis and healthy subjects. J Neuroimmunol 188(1–2):181–186. https://doi.org/10.1016/j.jneuroim.2007.06.012
Simpson S Jr, Stewart N, van der Mei I, Otahal P, Charlesworth J, Ponsonby AL, Blizzard L, Dwyer T et al (2015) Stimulated PBMC-produced IFN-gamma and TNF-alpha are associated with altered relapse risk in multiple sclerosis: results from a prospective cohort study. J Neurol Neurosurg Psychiatry 86(2):200–207. https://doi.org/10.1136/jnnp-2013-307336
Watad A, Azrielant S, Bragazzi NL, Sharif K, David P, Katz I, Aljadeff G, Quaresma M et al (2017) Seasonality and autoimmune diseases: the contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun 82:13–30. https://doi.org/10.1016/j.jaut.2017.06.001
Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC, Canadian Collaborative Study G (2005) Timing of birth and risk of multiple sclerosis: population based study. BMJ 330(7483):120. https://doi.org/10.1136/bmj.38301.686030.63
Rodriguez Cruz PM, Matthews L, Boggild M, Cavey A, Constantinescu CS, Evangelou N, Giovannoni G, Gray O et al (2016) Time- and region-specific season of birth effects in multiple sclerosis in the United Kingdom. JAMA Neurol 73(8):954–960. https://doi.org/10.1001/jamaneurol.2016.1463
Dobson R, Giovannoni G, Ramagopalan S (2013) The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry 84(4):427–432. https://doi.org/10.1136/jnnp-2012-303934
Jasper EA, Nidey NL, Schweizer ML, Ryckman KK (2020) Gestational vitamin D and offspring risk of multiple sclerosis: a systematic review and meta-analysis. Ann Epidemiol in press 43:11–17. https://doi.org/10.1016/j.annepidem.2019.12.010
Teuscher C, Bunn JY, Fillmore PD, Butterfield RJ, Zachary JF, Blankenhorn EP (2004) Gender, age, and season at immunization uniquely influence the genetic control of susceptibility to histopathological lesions and clinical signs of experimental allergic encephalomyelitis: implications for the genetics of multiple sclerosis. Am J Pathol 165(5):1593–1602. https://doi.org/10.1016/S0002-9440(10)63416-5
Reynolds JD, Case LK, Krementsov DN, Raza A, Bartiss R, Teuscher C (2017) Modeling month-season of birth as a risk factor in mouse models of chronic disease: from multiple sclerosis to autoimmune encephalomyelitis. FASEB J 31(6):2709–2719. https://doi.org/10.1096/fj.201700062
Teuscher C, Doerge RW, Fillmore PD, Blankenhorn EP (2006) eae36, a locus on mouse chromosome 4, controls susceptibility to experimental allergic encephalomyelitis in older mice and mice immunized in the winter. Genetics 172(2):1147–1153. https://doi.org/10.1534/genetics.105.049049
Álvarez-Sánchez N, Cruz-Chamorro I, López-Gónzalez A, Utrilla JC, Fernández-Santos JM, Martínez-López A, Lardone PJ, Guerrero JM et al (2015) Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun 50:101–114. https://doi.org/10.1016/j.bbi.2015.06.021
Carrillo-Vico A, Leech MD, Anderton SM (2010) Contribution of myelin autoantigen citrullination to T cell autoaggression in the central nervous system. J Immunol 184(6):2839–2846. https://doi.org/10.4049/jimmunol.0903639
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Noguchi K, Gel YR, Brunner E, Konietschke F (2012) nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. Journal of Statistical Software 50(12). https://doi.org/10.18637/jss.v050.i12
Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, Kumar V, Diavatopoulos DA et al (2016) Host and environmental factors influencing individual human cytokine responses. Cell 167(4):1111–1124 e1113. https://doi.org/10.1016/j.cell.2016.10.018
Aguirre-Gamboa R, Joosten I, Urbano PCM, van der Molen RG, van Rijssen E, van Cranenbroek B, Oosting M, Smeekens S et al (2016) Differential effects of environmental and genetic factors on T and B cell immune traits. Cell Rep 17(9):2474–2487. https://doi.org/10.1016/j.celrep.2016.10.053
Planelles D, Hernandez-Godoy J, Montoro A, Montoro J, Gonzalez-Molina A (1994) Seasonal variation in proliferative response and subpopulations of lymphocytes from mice housed in a constant environment. Cell Prolif 27(6):333–341. https://doi.org/10.1111/j.1365-2184.1994.tb01430.x
Ratajczak HV, Thomas PT, Sothern RB, Vollmuth T, Heck JD (1991) Reproducibility of seasonal effects in antibody formation and host resistance in the outbred mouse. Chronobiol Int 8(1):44–55. https://doi.org/10.3109/07420529109063918
Pati AK, Florentin I, Chung V, De Sousa M, Levi F, Mathe G (1987) Circannual rhythm in natural killer activity and mitogen responsiveness of murine splenocytes. Cell Immunol 108(1):227–234. https://doi.org/10.1016/0008-8749(87)90207-3
Juedes AE, Ruddle NH (2001) Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol 166(8):5168–5175. https://doi.org/10.4049/jimmunol.166.8.5168
Li J, Gran B, Zhang GX, Ventura ES, Siglienti I, Rostami A, Kamoun M (2003) Differential expression and regulation of IL-23 and IL-12 subunits and receptors in adult mouse microglia. J Neurol Sci 215(1–2):95–103. https://doi.org/10.1016/S0022-510X(03)00203-X
Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN (1998) Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol 84(2):238–249. https://doi.org/10.1016/S0165-5728(97)00208-7
Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE et al (2014) Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211(8):1533–1549. https://doi.org/10.1084/jem.20132477
Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D et al (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17(4):495–499. https://doi.org/10.1038/nm.2324
Werner P, Pitt D, Raine CS (2001) Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol 50(2):169–180. https://doi.org/10.1002/ana.1077
Hedstrom AK, Akerstedt T, Olsson T, Alfredsson L (2015) Shift work influences multiple sclerosis risk. Mult Scler 21(9):1195–1199. https://doi.org/10.1177/1352458514563592
Acknowledgments
We are grateful to María José Castro from the IBiS Flow Cytometry Facility, to Cristina Pichardo and the staff of the IBiS Animal Facility for their valuable assistance, and to John Leslie Brown for language assistance.
Availability of Data
Data used for this manuscript are available at https://doi.org/10.17632/j9427wpggx.3.
Funding
The work reported in the present manuscript was supported by the Department of Health of the Andalusian Government (PI-0209-2010, PI-0485-2014, and PC-0019-2017) and the PAIDI Program of the Andalusian Government (CTS160). NA-S was supported by fellowships from the National Net RETICEF for Aging Studies (Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad; RD06/0013/0001 and RD12/0043/0012; from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation) and by a fellowship from the Department of Health of the Andalusian Government (PC-0111-2016-0111), and IC-C was supported by an FPU grant from the Spanish Ministry of Education, Culture and Sport (FPU13/01210). The funding sources had no role in the design of the study, in the collection, analysis, and interpretation of data or in writing this manuscript.
Author information
Authors and Affiliations
Contributions
A.C-V., N.A.S., P.J.L., and J.M.G designed the study. N.A-S., I.C-C., A.I.A-L., A.L.G., and A.M-L. performed experiments and analyzed experimental data. J.R.L.R. performed statistical analyses. N.A-S and A.C.V. wrote the paper. All authors contributed to critically discuss the data and the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All necessary institutional and ethical review board approvals were obtained and the experiments were conducted in compliance with Spanish legislation and EU Directive 2010/63/EU for animal experiments.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 904 kb)
Rights and permissions
About this article
Cite this article
Álvarez-Sánchez, N., Cruz-Chamorro, I., Álvarez-López, A.I. et al. Seasonal Variations in Macrophages/Microglia Underlie Changes in the Mouse Model of Multiple Sclerosis Severity. Mol Neurobiol 57, 4082–4089 (2020). https://doi.org/10.1007/s12035-020-02017-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02017-x