Abstract
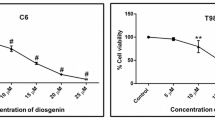
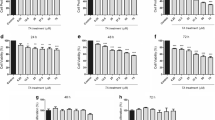
Gliomas are the most frequent type of primary brain tumor in adults. Their highly proliferative nature, complex cellular composition, and ability to escape therapies have confronted investigators for years, hindering the advancement toward an effective treatment. Agents that are safe and can be administered as dietary supplements have always remained priority to be most feasible for cancer therapy. Withania somnifera (ashwagandha) is an essential ingredient of Ayurvedic preparations and is known to eliminate cancer cells derived from a variety of peripheral tissues. Although our previous studies have addressed the in vitro anti-proliferative and differentiation-inducing properties of ashwagandha on neuronal cell lines, in vivo studies validating the same are lacking. While exploring the mechanism of its action in vitro, we observed that the ashwagandha water extract (ASH-WEX) induced the G2/M phase blockade and caused the activation of multiple pro-apoptotic pathways, leading to suppression of cyclin D1, bcl-xl, and p-Akt, and reduced the expression of polysialylated form of neural cell adhesion molecule (PSA-NCAM) as well as the activity of matrix metalloproteinases. ASH-WEX reduced the intracranial tumor volumes in vivo and suppressed the tumor-promoting proteins p-nuclear factor kappa B (NF-κB), p-Akt, vascular endothelial growth factor (VEGF), heat shock protein 70 (HSP70), PSA-NCAM, and cyclin D1 in the rat model of orthotopic glioma allograft. Reduction in glial fibrillary acidic protein (GFAP) and upregulation of mortalin and neural cell adhesion molecule (NCAM) expression specifically in tumor-bearing tissue further indicated the anti-glioma efficacy of ASH-WEX in vivo. Combining this enhanced understanding of the molecular mechanisms of ASH-WEX in glioma with in vivo model system offers new opportunities to develop therapeutic strategy for safe, specific, and effective formulations for treating brain tumors.






Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol 114(2):97–109. doi:10.1007/s00401-007-0243-4
Swartling FJ, Savov V, Persson AI, Chen J, Hackett CS, Northcott PA, Grimmer MR, Lau J et al (2012) Distinct neural stem cell populations give rise to disparate brain tumors in response to N-MYC. Cancer Cell 21(5):601–613. doi:10.1016/j.ccr.2012.04.012
Stupp R, Hegi ME, Gilbert MR, Chakravarti A (2007) Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol 25(26):4127–4136. doi:10.1200/JCO.2007.11.8554
Gerstner ER, Duda DG, di Tomaso E, Sorensen G, Jain RK, Batchelor TT (2007) Antiangiogenic agents for the treatment of glioblastoma. Expert Opin Investig Drugs 16(12):1895–1908. doi:10.1517/13543784.16.12.1895
Ohgaki H, Kleihues P (2007) Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170(5):1445–1453. doi:10.2353/ajpath.2007.070011
Singh B, Saxena AK, Chandan BK, Gupta DK, Bhutani KK, Anand KK (2001) Adaptogenic activity of a novel, withanolide-free aqueous fraction from the roots of Withania somnifera Dun. Phytother Res 15(4):311–318. doi:10.1002/ptr.858
Kulkarni SK, Dhir A (2008) Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry 32(5):1093–1105. doi:10.1016/j.pnpbp.2007.09.011
Kataria H, Shah N, Kaul SC, Wadhwa R, Kaur G (2011) Water extract of ashwagandha leaves limits proliferation and migration, and induces differentiation in glioma cells. Evid Based Complement Alternat Med. doi:10.1093/ecam/nep188
Kumar P, Singh R, Nazmi A, Lakhanpal D, Kataria H, Kaur G (2014) Glioprotective effects of ashwagandha leaf extract against lead induced toxicity. Biomed Res Int 2014:182029. doi:10.1155/2014/182029
Jayaprakasam B, Zhang Y, Seeram NP, Nair MG (2003) Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci 74(1):125–132
Widodo N, Kaur K, Shrestha BG, Takagi Y, Ishii T, Wadhwa R, Kaul SC (2007) Selective killing of cancer cells by leaf extract of ashwagandha: identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin Cancer Res 13(7):2298–2306. doi:10.1158/1078-0432.CCR-06-0948
Widodo N, Takagi Y, Shrestha BG, Ishii T, Kaul SC, Wadhwa R (2008) Selective killing of cancer cells by leaf extract of ashwagandha: components, activity and pathway analyses. Cancer Lett 262(1):37–47. doi:10.1016/j.canlet.2007.11.037
Widodo N, Priyandoko D, Shah N, Wadhwa R, Kaul SC (2010) Selective killing of cancer cells by ashwagandha leaf extract and its component withanone involves ROS signaling. PLoS One 5(10), e13536. doi:10.1371/journal.pone.0013536
Subbaraju GV, Vanisree M, Rao CV, Sivaramakrishna C, Sridhar P, Jayaprakasam B, Nair MG (2006) Ashwagandhanolide, a bioactive dimeric thiowithanolide isolated from the roots of Withania somnifera. J Nat Prod 69(12):1790–1792. doi:10.1021/np060147p
Shah N, Kataria H, Kaul SC, Ishii T, Kaur G, Wadhwa R (2009) Effect of the alcoholic extract of ashwagandha leaves and its components on proliferation, migration, and differentiation of glioblastoma cells: combinational approach for enhanced differentiation. Cancer Sci 100(9):1740–1747. doi:10.1111/j.1349-7006.2009.01236.x
Mishra LC, Singh BB, Dagenais S (2000) Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev 5(4):334–346
Winters M (2006) Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern Med Rev 11(4):269–277
Smilowitz HM, Weissenberger J, Weis J, Brown JD, O’Neill RJ, Laissue JA (2007) Orthotopic transplantation of v-src-expressing glioma cell lines into immunocompetent mice: establishment of a new transplantable in vivo model for malignant glioma. J Neurosurg 106(4):652–659. doi:10.3171/jns.2007.106.4.652
Wadhwa R, Singh R, Gao R, Shah N, Widodo N, Nakamoto T, Ishida Y, Terao K et al (2013) Water extract of ashwagandha leaves has anticancer activity: identification of an active component and its mechanism of action. PLoS One 8(10), e77189. doi:10.1371/journal.pone.0077189
Bryant MJ, Chuah TL, Luff J, Lavin MF, Walker DG (2008) A novel rat model for glioblastoma multiforme using a bioluminescent F98 cell line. J Clin Neurosci 15(5):545–551. doi:10.1016/j.jocn.2007.04.022
Karmakar S, Olive MF, Banik NL, Ray SK (2007) Intracranial stereotaxic cannulation for development of orthotopic glioblastoma allograft in Sprague-Dawley rats and histoimmunopathological characterization of the brain tumor. Neurochem Res 32(12):2235–2242. doi:10.1007/s11064-007-9450-6
Wilson TA, Karajannis MA, Harter DH (2014) Glioblastoma multiforme: state of the art and future therapeutics. Surg Neurol Int 5:64. doi:10.4103/2152-7806.132138
Toda M, Miura M, Asou H, Sugiyama I, Kawase T, Uyemura K (1999) Suppression of glial tumor growth by expression of glial fibrillary acidic protein. Neurochem Res 24(2):339–343
Wilhelmsson U, Eliasson C, Bjerkvig R, Pekny M (2003) Loss of GFAP expression in high-grade astrocytomas does not contribute to tumor development or progression. Oncogene 22(22):3407–3411. doi:10.1038/sj.onc.1206372
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7(3):309–316
Shih YY, Lee H, Nakagawara A, Juan HF, Jeng YM, Tsay YG, Lin DT, Hsieh FJ et al (2011) Nuclear GRP75 binds retinoic acid receptors to promote neuronal differentiation of neuroblastoma. PLoS One 6(10), e26236. doi:10.1371/journal.pone.0026236
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31(3):164–172. doi:10.1016/j.tibs.2006.01.006
Li G, Xu Y, Guan D, Liu Z, Liu DX (2011) HSP70 protein promotes survival of C6 and U87 glioma cells by inhibition of ATF5 degradation. J Biol Chem 286(23):20251–20259. doi:10.1074/jbc.M110.211771
Huerta S, Srivatsan ES, Venkatesan N, Peters J, Moatamed F, Renner S, Livingston EH (2001) Alternative mRNA splicing in colon cancer causes loss of expression of neural cell adhesion molecule. Surgery 130(5):834–843. doi:10.1067/msy.2001.116415
Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC et al (2002) NCAM regulates cell motility. J Cell Sci 115(Pt 2):283–292
Edvardsen K, Chen W, Rucklidge G, Walsh FS, Obrink B, Bock E (1993) Transmembrane neural cell-adhesion molecule (NCAM), but not glycosyl-phosphatidylinositol-anchored NCAM, down-regulates secretion of matrix metalloproteinases. Proc Natl Acad Sci U S A 90(24):11463–11467
Edvardsen K, Pedersen PH, Bjerkvig R, Hermann GG, Zeuthen J, Laerum OD, Walsh FS, Bock E (1994) Transfection of glioma cells with the neural-cell adhesion molecule NCAM: effect on glioma-cell invasion and growth in vivo. Int J Cancer 58(1):116–122
Tanaka F, Otake Y, Nakagawa T, Kawano Y, Miyahara R, Li M, Yanagihara K, Nakayama J et al (2000) Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res 60(11):3072–3080
Gallagher HC, Odumeru OA, Regan CM (2000) Regulation of neural cell adhesion molecule polysialylation state by cell-cell contact and protein kinase C delta. J Neurosci Res 61(6):636–645. doi:10.1002/1097-4547(20000915)61
Kontogianni K, Nicholson AG, Butcher D, Sheppard MN (2005) CD56: a useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artefact. J Clin Pathol 58(9):978–980. doi:10.1136/jcp.2004.023044
Ray JM, Stetler-Stevenson WG (1994) The role of matrix metalloproteases and their inhibitors in tumor invasion, metastasis and angiogenesis. Eur Respir J 7(11):2062–2072
Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89(17):1260–1270
Sawaya R, Go Y, Kyritisis AP, Uhm J, Venkaiah B, Mohanam S, Gokaslan ZL, Rao JS (1998) Elevated levels of Mr 92,000 type IV collagenase during tumor growth in vivo. Biochem Biophys Res Commun 251(2):632–636. doi:10.1006/bbrc.1998.9466
Ulasov I, Yi R, Guo D, Sarvaiya P, Cobbs C (2014) The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochim Biophys Acta 1846(1):113–120. doi:10.1016/j.bbcan.2014.03.002
Cohen AL, Colman H (2015) Glioma biology and molecular markers. Cancer Treat Res 163:15–30. doi:10.1007/978-3-319-12048-5_2
Mathur R, Gupta SK, Singh N, Mathur S, Kochupillai V, Velpandian T (2006) Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J Ethnopharmacol 105(3):336–341. doi:10.1016/j.jep.2005.11.020
Gao R, Shah N, Lee JS, Katiyar SP, Li L, Oh E, Sundar D, Yun CO et al (2014) Withanone-rich combination of ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol Cancer Ther 13(12):2930–2940. doi:10.1158/1535-7163.MCT-14-0324
Dole MG, Jasty R, Cooper MJ, Thompson CB, Nunez G, Castle VP (1995) Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res 55(12):2576–2582
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ (1998) Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A 95(10):5724–5729
Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N (1997) The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11(6):701–713
Lawlor MA, Alessi DR (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114(Pt 16):2903–2910
Zhang X, Chen T, Zhang J, Mao Q, Li S, Xiong W, Qiu Y, Xie Q et al (2012) Notch1 promotes glioma cell migration and invasion by stimulating beta-catenin and NF-kappaB signaling via AKT activation. Cancer Sci 103(2):181–190. doi:10.1111/j.1349-7006.2011.02154.x
Oh JH, Kwon TK (2009) Withaferin A inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules by inactivation of Akt and NF-kappaB in human pulmonary epithelial cells. Int Immunopharmacol 9(5):614–619. doi:10.1016/j.intimp.2009.02.002
Li X, Zhu F, Jiang J, Sun C, Wang X, Shen M, Tian R, Shi C et al (2014) Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer Lett. doi:10.1016/j.canlet.2014.11.026
Donnellan R, Chetty R (1998) Cyclin D1 and human neoplasia. Mol Pathol 51(1):1–7
Molenaar JJ, Ebus ME, Koster J, van Sluis P, van Noesel CJ, Versteeg R, Caron HN (2008) Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer Res 68(8):2599–2609. doi:10.1158/0008-5472.CAN-07-5032
Sallinen SL, Sallinen PK, Kononen JT, Syrjakoski KM, Nupponen NN, Rantala IS, Helen PT, Helin HJ et al (1999) Cyclin D1 expression in astrocytomas is associated with cell proliferation activity and patient prognosis. J Pathol 188(3):289–293. doi:10.1002/(SICI)1096-9896(199907)188:3<289::AID-PATH351>3.0.CO;2-X
Kataria H, Wadhwa R, Kaul SC, Kaur G (2013) Withania somnifera water extract as a potential candidate for differentiation based therapy of human neuroblastomas. PLoS One 8(1), e55316. doi:10.1371/journal.pone.0055316
Owens GC, Orr EA, DeMasters BK, Muschel RJ, Berens ME, Kruse CA (1998) Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res 58(9):2020–2028
Arato-Ohshima T, Sawa H (1999) Over-expression of cyclin D1 induces glioma invasion by increasing matrix metalloproteinase activity and cell motility. Int J Cancer 83(3):387–392. doi:10.1002/(SICI)1097-0215(19991029)83:3<387::AID-IJC15>3.0.CO;2-O
Pu P, Kang C, Li J, Jiang H (2004) Antisense and dominant-negative AKT2 cDNA inhibits glioma cell invasion. Tumor Biol 25(4):172–178. doi:10.1159/000081099
Pu P, Kang C, Li J, Jiang H, Cheng J (2006) The effects of antisense AKT2 RNA on the inhibition of malignant glioma cell growth in vitro and in vivo. J Neurooncol 76(1):1–11. doi:10.1007/s11060-005-3029-3
Barkett M, Gilmore TD (1999) Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18(49):6910–6924. doi:10.1038/sj.onc.1203238
Baud V, Karin M (2009) Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8(1):33–40. doi:10.1038/nrd2781
Xie TX, Xia Z, Zhang N, Gong W, Huang S (2010) Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep 23(3):725–732
Wang CN, Shiao YJ, Lin YL, Chen CF (1999) Nepalolide A inhibits the expression of inducible nitric oxide synthase by modulating the degradation of IkappaB-alpha and IkappaB-beta in C6 glioma cells and rat primary astrocytes. Br J Pharmacol 128(2):345–356. doi:10.1038/sj.bjp.0702785
Ansari SA, Safak M, Del Valle L, Enam S, Amini S, Khalili K (2001) Cell cycle regulation of NF-kappa b-binding activity in cells from human glioblastomas. Exp Cell Res 265(2):221–233. doi:10.1006/excr.2001.5168
Nagai S, Washiyama K, Kurimoto M, Takaku A, Endo S, Kumanishi T (2002) Aberrant nuclear factor-kappaB activity and its participation in the growth of human malignant astrocytoma. J Neurosurg 96(5):909–917. doi:10.3171/jns.2002.96.5.0909
Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB (2006) Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther 5(6):1434–1445. doi:10.1158/1535-7163.MCT-06-0096
Patil D, Gautam M, Mishra S, Karupothula S, Gairola S, Jadhav S, Pawar S, Patwardhan B (2013) Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J Pharm Biomed Anal 80:203–212. doi:10.1016/j.jpba.2013.03.001
Vareed SK, Bauer AK, Nair KM, Liu Y, Jayaprakasam B, Nair MG (2014) Blood-brain barrier permeability of bioactive withanamides present in Withania somnifera fruit extract. Phytother Res 28(8):1260–1264. doi:10.1002/ptr.5118
Kataria H, Gupta M, Lakhman S, Kaur G (2015) Withania somnifera aqueous extract facilitates the expression and release of GnRH: in vitro and in vivo study. Neurochem Int (in press)
Acknowledgments
Infrastructure provided by the University Grants Commission (UGC), India, under UPE and CPEPA schemes, and the Department of Biotechnology (DBT), India, under DISC facility, is highly acknowledged. This study was funded by DBT project grant to GK and fellowship grant to SK. HK is thankful to the Council of Scientific and Industrial Research (CSIR) for providing fellowship grant during the course of this study.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataria, H., Kumar, S., Chaudhary, H. et al. Withania somnifera Suppresses Tumor Growth of Intracranial Allograft of Glioma Cells. Mol Neurobiol 53, 4143–4158 (2016). https://doi.org/10.1007/s12035-015-9320-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9320-1