Abstract
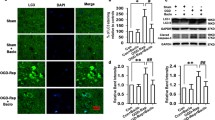
Hyperpolarization-activated cyclic-nucleotide-gated cation nonselective (HCN) channels are involved in the pathology of nervous system diseases. HCN channels and γ-aminobutyric acid (GABA) receptors can mutually co-regulate the function of neurons in many brain areas. However, little is known about the co-regulation of HCN channels and GABA receptors in the chronic ischemic rats with possible features of vascular dementia. Protein kinase A (PKA) and TPR containing Rab8b interacting protein (TRIP8b) can modulate GABAB receptors cell surface stability and HCN channel trafficking, respectively, and adaptor-associated kinase 1 (AAK1) inhibits the function of the major TRIP8b-interacting protein adaptor protein 2 (AP2) via phosphorylating the AP2 μ2 subunit. Until now, the role of these regulatory factors in chronic cerebral hypoperfusion is unclear. In the present study, we evaluated whether and how HCN channels and GABAB receptors were pathologically altered and investigated neuroprotective effects of GABAB receptors activation and cross-talk networks between GABAB receptors and HCN channels in the hippocampal CA1 area in chronic cerebral hypoperfusion rat model. We found that cerebral hypoperfusion for 5 weeks by permanent occlusion of bilateral common carotid arteries (two-vessel occlusion, 2VO) induced marked spatial and nonspatial learning and memory deficits, significant neuronal loss and decrease in dendritic spine density, impairment of long-term potentiation (LTP) at the Schaffer collateral-CA1 synapses, and reduction of surface expression of GABAB R1, GABAB R2, and HCN1, but increase in HCN2 surface expression. Meanwhile, the protein expression of TRIP8b (1a-4), TRIP8b (1b-2), and AAK1 was significantly decreased. Baclofen, a GABAB receptor agonist, markedly improved the memory impairment and alleviated neuronal damage. Besides, baclofen attenuated the decrease of surface expression of GABAB R1, GABAB R2, and HCN1, but downregulated HCN2 surface expression. Furthermore, baclofen could restore expression of AAK1 protein and significantly increase p-PKA, TRIP8b (1a-4), TRIP8b (1b-2), and p-AP2 μ2 expression. Those findings suggested that, under chronic cerebral hypoperfusion, activation of PKA could attenuate baclofen-induced decrease in surface expression of GABAB R1 and GABAB R2, and activation of GABAB receptors not only increased the expression of TRIP8b (1a-4) and TRIP8b (1b-2) but also regulated the function of TRIP8b via AAK1 and p-AP2 μ2, which restored the balance of HCN1/HCN2 surface expression in rat hippocampal CA1 area, and thus ameliorated cognitive impairment.







Similar content being viewed by others
References
Halliwell JV, Adams PR (1982) Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res 250(1):71–92
Wahl-Schott C, Biel M (2009) HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66(3):470–494. doi:10.1007/s00018-008-8525-0
Biel M, Wahl-Schott C, Michalakis S, Zong X (2009) Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89(3):847–885. doi:10.1152/physrev.00029.2008
Doan TN, Kunze DL (1999) Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol 514(Pt 1):125–138
Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A (2004) A behavioral role for dendritic integration: HCN1 channels constrain spatial inputs to distal dendrites memory and plasticity at of CA1 pyramidal neurons. Cell 119(5):719–732. doi:10.1016/j.cell.2004.11.020
Huang CC, Hsu KS (2003) Reexamination of the role of hyperpolarization-activated cation channels in short- and long-term plasticity at hippocampal mossy fiber synapses. Neuropharmacology 44(7):968–981. doi:10.1016/S0028-3908(03)00098-4
Mellor J, Nicoll RA, Schmitz D (2002) Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science 295(5552):143–147. doi:10.1126/science.1064285
Leresche N, Lightowler S, Soltesz I, Jassik-Gerschenfeld D, Crunelli V (1991) Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol 441:155–174
Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ (2001) Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1–4 suggests evolving roles in the developing rat hippocampus. Neuroscience 106(4):689–698. doi:10.1016/S0306-4522(01)00314-1
Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin DQ, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A (2003) The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115(5):551–564. doi:10.1016/S0092-8674(03)00884-5
Li S, He Z, Guo L, Huang L, Wang J, He W (2010) Behavioral alterations associated with a down regulation of HCN1 mRNA in hippocampal cornus ammon 1 region and neocortex after chronic incomplete global cerebral ischemia in rats. Neuroscience 165(3):654–661. doi:10.1016/j.neuroscience.2009.10.053
Postea O, Biel M (2011) Exploring HCN channels as novel drug targets. Nat Rev Drug Discov 10(12):903–914. doi:10.1038/Nrd3576
Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22(2):216–224. doi:10.1093/emboj/cdg032
Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA (2011) HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 333(6048):1462–1466. doi:10.1126/science.1206243
Notomi T, Shigemoto R (2004) Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471(3):241–276. doi:10.1002/cne.11039
Chen X, Shu S, Schwartz LC, Sun C, Kapur J, Bayliss DA (2010) Homeostatic regulation of synaptic excitability: tonic GABA(A) receptor currents replace I(h) in cortical pyramidal neurons of HCN1 knock-out mice. J Neurosci 30(7):2611–2622. doi:10.1523/JNEUROSCI.3771-09.2010
Bonin RP, Zurek AA, Yu JY, Bayliss DA, Orser BA (2013) Hyperpolarization-activated current (I-h) is reduced in hippocampal neurons from Gabra5−/− mice. PloS One 8(3):e58679. doi:10.1371/journal.pone.0058679
Atherton JF, Kitano K, Baufreton J, Fan K, Wokosin D, Tkatch T, Shigemoto R, Surmeier DJ, Bevan MD (2010) Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus. J Neurosci 30(47):16025–16040. doi:10.1523/Jneurosci.3898-10.2010
Watts AE, Williams JT, Henderson G (1996) Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J Neurophysiol 76(4):2262–2270
Chebib M, Johnston GA (2000) GABA-Activated ligand gated ion channels: medicinal chemistry and molecular biology. J Med Chem 43(8):1427–1447
Jiang ZG, Pessia M, North RA (1993) Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J Physiol 462:753–764
Sternau LL, Lust WD, Ricci AJ, Ratcheson R (1989) Role for gamma-aminobutyric acid in selective vulnerability in gerbils. Stroke 20(2):281–287
Lal S, Shuaib A, Ijaz S (1995) Baclofen is cytoprotective to cerebral ischemia in gerbils. Neurochem Res 20(2):115–119
Zhang F, Li C, Wang R, Han D, Zhang QG, Zhou C, Yu HM, Zhang GY (2007) Activation of GABA receptors attenuates neuronal apoptosis through inhibiting the tyrosine phosphorylation of NR2A by Src after cerebral ischemia and reperfusion. Neuroscience 150(4):938–949. doi:10.1016/j.neuroscience.2007.09.070
Xu J, Li C, Yin XH, Zhang GY (2008) Additive neuroprotection of GABA A and GABA B receptor agonists in cerebral ischemic injury via PI-3 K/Akt pathway inhibiting the ASK1-JNK cascade. Neuropharmacology 54(7):1029–1040. doi:10.1016/j.neuropharm.2008.01.014
Zhou C, Li C, Yu HM, Zhang F, Han D, Zhang GY (2008) Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J Neurosci Res 86(13):2973–2983. doi:10.1002/jnr.21728
Tuttolomondo A, Di Sciacca R, Di Raimondo D, Arnao V, Renda C, Pinto A, Licata G (2009) Neuron protection as a therapeutic target in acute ischemic stroke. Curr Top Med Chem 9(14):1317–1334
Jackson-Friedman C, Lyden PD, Nunez S, Jin A, Zweifler R (1997) High dose baclofen is neuroprotective but also causes intracerebral hemorrhage: a quantal bioassay study using the intraluminal suture occlusion method. Exp Neurol 147(2):346–352. doi:10.1006/exnr.1997.6637
Cimarosti H, Kantamneni S, Henley JM (2009) Ischaemia differentially regulates GABA(B) receptor subunits in organotypic hippocampal slice cultures. Neuropharmacology 56(8):1088–1096. doi:10.1016/j.neuropharm.2009.03.007
Costa C, Leone G, Saulle E, Pisani F, Bernardi G, Calabresi P (2004) Coactivation of GABA(A) and GABA(B) receptor results in neuroprotection during in vitro ischemia. Stroke 35(2):596–600. doi:10.1161/01.STR.0000113691.32026.06
Santoro B, Wainger BJ, Siegelbaum SA (2004) Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci 24(47):10750–10762. doi:10.1523/JNEUROSCI.3300-04.2004
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2(2):107–117. doi:10.1038/35052055
Piskorowski R, Santoro B, Siegelbaum SA (2011) TRIP8b splice forms act in concert to regulate the localization and expression of HCN1 channels in CA1 pyramidal neurons. Neuron 70(3):495–509. doi:10.1016/j.neuron.2011.03.023
Santoro B, Piskorowski RA, Pian P, Hu L, Liu HY, Siegelbaum SA (2009) TRIP8b splice variants form a family of auxiliary subunits that regulate gating and trafficking of HCN channels in the brain. Neuron 62(6):802–813. doi:10.1016/j.neuron.2009.05.009
Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM (2009) Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci 29(19):6250–6265. doi:10.1523/JNEUROSCI.0856-09.2009
Zolles G, Wenzel D, Bildl W, Schulte U, Hofmann A, Muller CS, Thumfart JO, Vlachos A, Deller T, Pfeifer A, Fleischmann BK, Roeper J, Fakler B, Klocker N (2009) Association with the auxiliary subunit PEX5R/Trip8b controls responsiveness of HCN Channels to cAMP and adrenergic stimulation. Neuron 62(6):814–825. doi:10.1016/j.neuron.2009.05.008
Popova NV, Plotnikov AN, Ziganshin R, Deyev IE, Petrenko AG (2008) Analysis of proteins interacting with TRIP8b adapter. Biochemistry (Mosc) 73(6):644–651
Santoro B, Hu L, Liu HY, Saponaro A, Pian P, Piskorowski RA, Moroni A, Siegelbaum SA (2011) TRIP8b regulates HCN1 channel trafficking and gating through two distinct C-terminal interaction sites. J Neurosci 31(11):4074–4086. doi:10.1523/Jneurosci.5707-10.2011
Kirchhausen T (1999) Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol 15:705–732. doi:10.1146/annurev.cellbio.15.1.705
Gu M, Liu Q, Watanabe S, Sun L, Hollopeter G, Grant BD, Jorgensen EM (2013) AP2 hemicomplexes contribute independently to synaptic vesicle endocytosis. Elife 2:e00190. doi:10.7554/eLife.00190
Conner SD, Schmid SL (2002) Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol 156(5):921–929. doi:10.1083/jcb.200108123
Fairfax BP, Pitcher JA, Scott MG, Calver AR, Pangalos MN, Moss SJ, Couve A (2004) Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J Biol Chem 279(13):12565–12573. doi:10.1074/jbc.M311389200
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Block F (1999) Global ischemia and behavioural deficits. Prog Neurobiol 58(3):279–295
Benice TS, Raber J (2008) Object recognition analysis in mice using nose-point digital video tracking. J Neurosci Methods 168(2):422–430. doi:10.1016/j.jneumeth.2007.11.002
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110. doi:10.1007/s10339-011-0430-z
Okuda S, Roozendaal B, McGaugh JL (2004) Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A 101(3):853–858. doi:10.1073/pnas.0307803100
Li CJ, Zhou M, Li HG, Lv Q, Xu XL, Guo LJ (2013) Clonidine suppresses the induction of long-term potentiation by inhibiting HCN channels at the Schaffer collateral-CA1 synapse in anesthetized adult rats. Cell Mol Neurobiol 33(8):1075–1086. doi:10.1007/s10571-013-9974-z
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361(6407):31–39. doi:10.1038/361031a0
Desestret V, Riou A, Chauveau F, Cho TH, Devillard E, Marinescu M, Ferrera R, Rey C, Chanal M, Angoulvant D, Honnorat J, Nighoghossian N, Berthezene Y, Nataf S, Wiart M (2013) In vitro and in vivo models of cerebral ischemia show discrepancy in therapeutic effects of M2 macrophages. PloS One 8(6):e67063. doi:10.1371/journal.pone.0067063
Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK (2005) Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience 133(2):463–470. doi:10.1016/j.neuroscience.2005.02.021
Alquicer G, Morales-Medina JC, Quirion R, Flores G (2008) Postweaning social isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. J Chem Neuroanat 35(2):179–187. doi:10.1016/j.jchemneu.2007.10.001
Powell KL, Ng C, O’Brien TJ, Xu SH, Williams DA, Foote SJ, Reid CA (2008) Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia 49(10):1686–1695. doi:10.1111/j.1528-1167.2008.01593.x
Arolfo MP, Zanudio MA, Ramirez OA (1998) Baclofen infused in rat hippocampal formation impairs spatial learning. Hippocampus 8(2):109–113. doi:10.1002/(SICI)1098-1063(1998)8:2<109::AID-HIPO2>3.0.CO;2-G
McNamara RK, Skelton RW (1996) Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav 53(2):303–308
Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S (2009) GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K + channels. Neuron 63(2):230–243. doi:10.1016/j.neuron.2009.06.022
Nakagawa Y, Takashima T (1997) The GABA(B) receptor antagonist CGP36742 attenuates the baclofen- and scopolamine-induced deficit in Morris water maze task in rats. Brain Res 766(1–2):101–106
Sarti C, Pantoni L, Bartolini L, Inzitari D (2002) Persistent impairment of gait performances and working memory after bilateral common carotid artery occlusion in the adult Wistar rat. Behav Brain Res 136(1):13–20
Kim SK, Cho KO, Kim SY (2008) White matter damage and Hippocampal neurodegeneration induced by permanent bilateral occlusion of common carotid artery in the rat: comparison between wistar and Sprague–Dawley strain. Korean J Physiol Pharmacol 12(3):89–94. doi:10.4196/kjpp.2008.12.3.89
Pitsikas N, Rigamonti AE, Cella SG, Muller EE (2003) The GABAB receptor and recognition memory: possible modulation of its behavioral effects by the nitrergic system. Neuroscience 118(4):1121–1127
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54(1):162–180. doi:10.1016/j.brainresrev.2007.01.003
von Bohlen, Halbach O (2009) Structure and function of dendritic spines within the hippocampus. Ann Anat 191(6):518–531. doi:10.1016/j.aanat.2009.08.006
Jia H, Zhang XM, Zhang BA, Liu Y, Li JM (2012) Dendritic morphology of neurons in medial prefrontal cortex and hippocampus in 2VO rats. Neurol Sci 33(5):1063–1070. doi:10.1007/s10072-011-0898-4
Anju TR, Jayanarayanan S, Paulose CS (2011) Decreased GABAB receptor function in the cerebellum and brain stem of hypoxic neonatal rats: role of glucose, oxygen and epinephrine resuscitation. J Biomed Sci 18:31. doi:10.1186/1423-0127-18-31
Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA (2000) Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20(14):5264–5275
Santoro B, Baram TZ (2003) The multiple personalities of h-channels. Trends Neurosci 26(10):550–554. doi:10.1016/j.tins.2003.08.003
Brewster A, Bender RA, Chen YC, Dube C, Eghbal-Ahmadi M, Baram TZ (2002) Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci 22(11):4591–4599
Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65:453–480. doi:10.1146/annurev.physiol.65.092101.142734
Brewster AL, Bernard JA, Gall CM, Baram TZ (2005) Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis 19(1–2):200–207. doi:10.1016/J.Nbd.12.015
Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S (2002) Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol 156(5):791–795. doi:10.1083/jcb.200111068
Feinklestein SP, Fisher M, Furland AJ, Goldstein LB, Gorelick PB, Kaste M, Lees KR, Traystman RJ, Albers GW, Anwer UE, Ashwood T, Barone FC, Basta SL, Bogousslavsky J, Buchan AM, Cady WJ, Chan PH, Clemens JA, Cox BF, Craddock RE, Cramer SC, del Zoppo GJ, Dielrich WD, Elliott P, Faden AI, Feuerstein GZ, Ginsberg MD, Gold M, Greene WL, Hall ED, Hsu CY, Hunter AJ, Lai M, Lesko LM, Levy DE, Li FH, Locke KW, Lodge D, Lowe D, Marcoux FW, McCulloch J, McDermott J, Meibach R, Messersmith EK, Moseley M, Moskowitz MA, Mueller AL, Munro F, Nudo RJ, Oeda J, Ohlstein EH, Parsons A, Patmore L, Poole RM, Pschorn U, Pulsinelli WA, Sacco RL, Saeki S, Salazar-Grueso E, Sandage BW, Schallert T, Schielke GP, Sharkey J, Sotak CH, Steiger B, Storall S, Takahashi Y, Tumas D, Van Bruggen N, Versavel M, Vornov J, Walker MD, Wallin B, Wang J, Warach S, Wells DS, Witcher JA, Round STAI (1999) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30(12):2752–2758
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC, No. 81173038) to Lianjun Guo.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chang-jun Li and Yun Lu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Cj., Lu, Y., Zhou, M. et al. Activation of GABAB Receptors Ameliorates Cognitive Impairment via Restoring the Balance of HCN1/HCN2 Surface Expression in the Hippocampal CA1 Area in Rats With Chronic Cerebral Hypoperfusion. Mol Neurobiol 50, 704–720 (2014). https://doi.org/10.1007/s12035-014-8736-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8736-3