Abstract
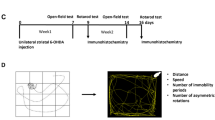
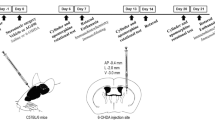
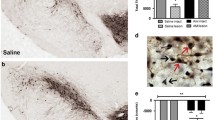
Excess glutamatergic neurotransmission may contribute to excitotoxic loss of nigrostriatal neurons in Parkinson's disease (PD). Here, we determined if increasing glutamate uptake could reduce the extent of tyrosine hydroxylase (TH) loss in PD progression. The beta-lactam antibiotic, ceftriaxone, increases the expression of glutamate transporter 1 (GLT-1), a glutamate transporter that plays a major role in glutamate clearance in central nervous system and may attenuate adverse behavioral or neurobiological function in other neurodegenerative disease models. In association with >80 % TH loss, we observed a significant decrease in glutamate uptake in the established 6-hydroxydopamine (6-OHDA) PD model. Ceftriaxone (200 mg/kg, i.p.) increased striatal glutamate uptake with >5 consecutive days of injection in nonlesioned rats and lasted out to 14 days postinjection, a time beyond that required for 6-OHDA to produce >70 % TH loss (∼9 days). When ceftriaxone was given at the time of 6-OHDA, TH loss was ∼57 % compared to ∼85 % in temporally matched vehicle-injected controls and amphetamine-induced rotation was reduced about 2-fold. This attenuation of TH loss was associated with increased glutamate uptake, increased GLT-1 expression, and reduced Serine 19 TH phosphorylation, a calcium-dependent target specific for nigrostriatal neurons. These results reveal that glutamate uptake can be targeted in a PD model, decrease the rate of TH loss in a calcium-dependent manner, and attenuate locomotor behavior associated with 6-OHDA lesion. Given that detection of reliable PD markers will eventually be employed in susceptible populations, our results give credence to the possibility that increasing glutamate uptake may prolong the time period before locomotor impairment occurs.








Similar content being viewed by others
References
Fonnum F (1984) Glutamate: a neurotransmitter in the mammalian brain. J Neurochem 42:1–11
Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB (2002) Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain 125:1908–1922
Lievens JC, Salin P, Nieoullon A, Kerkerian-Le Goff L (2001) Nigrostriatal denervation does not affect glutamate transporter mRNA expression but subsequent levodopa treatment selectively increases GLT1 mRNA and protein expression in the rat striatum. J Neurochem 79:893–902
Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR et al (2008) Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience 153:329–337
Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW (1995) Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol 38:73–84
Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R et al (2011) Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol 226:2484–2493
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Albin RL, Young AB, Penney JB (1995) The functional anatomy of disorders of the basal ganglia. Trends Neurosci 18:63–64
Chung EK, Chen LW, Chan YS, Yung KK (2008) Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J Comp Neurol 511:421–437
Hazell AS, Itzhak Y, Liu H, Norenberg MD (1997) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) decreases glutamate uptake in cultured astrocytes. J Neurochem 68:2216–2219
Plaitakis A, Shashidharan (2000) Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson's disease. J Neurol 247(Suppl 2):S1125–S1135
Lindefors N, Ungerstedt U (1990) Bilateral regulation of glutamate tissue and extra- cellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci Lett 115:248–252
Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF (1999) Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience 88:1–16
Robinson S, Freeman P, Moore C, Touchon JC, Krentz L, Meshul CK (2003) Acute and subchronic MPTP administration differentially affects striatal glutamate synaptic function. Exp Neurol 180:74–87
Touchon JC, Holmer HK, Moore C, McKee BL, Frederickson J, Meshul CK (2005) Apomorphine induced alterations in striatal and substantia nigra pars reticulata glutamate following unilateral loss of striatal dopamine. Exp Neurol 193:131–140
Meredith GE, Totterdell S, Beales M, Meshul CK (2009) Impaired glutamate ho- meostasis and programmed cell death in a chronic MPTP mouse model of Parkinson's disease. Exp Neurol 219:334–340
Weihmuller FB, Ulas J, Nguyen L, Cotman CW, Marshall JF (1992) Elevated NMDA receptors in parkinsonian striatum. Neuroreport 3:977–980
Ulas J, Weihmuller FB, Brunner LC, Joyce JN, Marshall JF, Cotman CW (1994) Selective increase of NMDA-sensitive glutamate binding in the striatum of Parkinson's disease, Alzheimer's disease, and mixed Parkinson's disease/Alzheimer's disease patients. J Neurosci 14:6317–6324
Sanchez-Pernaute R, Wang JQ, Kuruppu D, Cao L, Tueckmantel W, Kozikowski A et al (2008) Enhanced binding of metabotropic glutamate receptor type 5 (mGluR5) PET tracers in the brain of parkinsonian primates. Neuroimage 42:248–251
Faden AI, Demediuk P, Panter SS, Vink R (1989) The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244:789–800
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622
Choi D (1985) Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett 58:293–297
Meldrum B, Garthwaite J (1990) Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 11:379–387
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542
Geddes JW, Saatman KE (2010) Targeting individual calpain isoforms for neuroprotection. Exp Neurol 226:6–7
Grant RJ, Sellings LH, Crocker SJ, Melloni E, Park DS, Clarke PB (2009) Effects of calpain inhibition on dopaminergic markers and motor function following intrastriatal 6-hydroxydopamine administration in rats. Neuroscience 158:558–569
Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS et al (2010) Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain 133:808–821
Ambrosi G, Armentero MT, Levandis G, Bramanti P, Nappi G, Blandini F (2010) Effects of early and delayed treatment with an mGluR5 antagonist on motor impairment, nigrostriatal damage and neuroinflammation in a rodent model of Parkinson's disease. Brain Res Bull 82:29–38
Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F (2006) Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson's disease. Neurobiol Dis 22:1–9
Vernon AC, Zbarsky V, Datla KP, Croucher MJ, Dexter DT (2007) Subtype selective antagonism of substantia nigra pars compacta Group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J Neurochem 103:1075–1091
Hallert PJ, Standaert DG (2004) Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Ther 102:155–174
Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW (2008) Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord 15:1860–1866
Hubsher G, Haider M, Okun MS (2012) Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology 78:1096–1099
Lipton SA (2006) Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov 5:160–170
Koller M, Urwyler S (2006) Novel N-methyl-D-aspartate receptor antagonists: a review of compounds patented since 2006. Expert Opin Ther Pat 20:1683–1702
Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14
Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW et al (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K et al (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Selkirk JV, Nottebaum LM, Vana AM, Verge GM, Mackay KB, Stiefel TH et al (2005) Role of the GLT-1 subtype of glutamate transporter in glutamate homeostasis: the GLT-1-preferring inhibitor WAY-855 produces marginal neurotoxicity in the rat hippocampus. Eur J Neurosci 21:3217–3228
Duan S, Anderson CM, Stein BA, Swanson RA (1999) Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19:10193–10200
Salvatore MF, Davis RW, Arnold JC, Chotibut T (2012) Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser19. Exp Neur 234:428–436
Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE et al (2005) Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433:73–77
van Landeghem FK, Weiss T, Oehmichen M, von Deimling A (2006) Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. J Neurotrauma 23:1518–1528
Rao VL, Dogan A, Bowen KK et al (2001) Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur J Neurosci 13:119–28
Hirsch EC (2000) Nigrostriatal system plasticity in Parkinson's disease: effect of dopaminergic denervation and treatment. Ann Neurol 47:S115–20
Smeyne RJ, Jackson-Lewis V (2005) The MPTP model of Parkinson's disease. Brain Res Mol Brain Res 134:57–66
Leung TC, Lui CN, Chen LW et al (2012) Ceftriaxone ameliorates motor deficits and protects dopaminergic neurons in 6-hydroxydopamine-lesioned rats. ACS Chem Neurosci 3:22–30
Sari Y, Prieto A, Barton SJ et al (2010) Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington's disease. J Biomed Sci 17:62
Brown RH Jr (2005) Amyotrophic lateral sclerosis—a new role for old drugs. N Engl J Med 352:1376–1378
Chotibut T, Apple DM, Jefferis R et al (2012) Dopamine transporter loss in 6-OHDA Parkinson's model is unmet by parallel reduction in dopamine uptake. PLos One. doi:10.1371/journal.pone.0052322
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic, Sydney
Hudson JL, van Horne CG, Stromberg I, Brock S, Clayton J, Masserano J et al (1993) Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 626:167–174
Salvatore MF, Waymire JC, Haycock JW (2001) Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J Neurochem 79:349–360
Salvatore MF, Apparsundaram S, Gerhardt GA (2003) Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol Aging 24:1147–1154
Henn FA, Anderson DJ, Rustad DG (1976) Glial contamination of synaptosomal fractions. Brain Res 101:341–344
Suchak SK, Baloyianni NV, Perkinton MS, Williams RJ, Meldrum BS, Rattray M (2003) The ‘glial’ glutamate transporter, EAAT2 (Glt-1) accounts for high affinity glutamate uptake into adult rodent nerve endings. J Neurochem 84:522–532
Minger SL, Geddes JW, Holtz ML, Craddock SD, Whiteheart SW, Siman RG, Pettigrew LC (1998) Glutamate receptor antagonists inhibit calpain-mediated cytoskeletal proteolysis in focal cerebral ischemia 810:181–199
Nickell J, Pomerleau F, Allen J, Gerhardt GA (2005) Age-related changes in the dy- namics of potassium-evoked L-glutamate release in the striatum of Fisher 344 rats. J Neural Transm 112:87–96
Wassum KM, Tolosa VM, Wang J, Walker E, Monbouquette HG, Maidment NT (2008) Silicon wafer-based platinum microelectrode array biosensor for near real- time measurement of glutamate in vivo. Sens Basel Sens 8:5023–5036
Salvatore MF, Pruett BS, Dempsey C, Fields V (2012) Comprehensive profiling of dopamine regulation in substantia nigra and ventral tegmental area. J Vis Exp. doi:10.3791/4171
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Nakashima A, Mori K, Kaneko YS, Nobuhiro H, Toshiharu N, Ota A (2011) Phosphorylation of the N-terminal portion of tyrosine hydroxylase triggers proteasomal digestion of the enzyme. Bio Biophys Res Com 407:343–347
DiFiglia M (1990) Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci 13:286–289
Ross CA (2002) Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington's disease and related disorders. Neuron 35:819–822
Andre VM, Cepeda C, Levine MS (2010) Dopamine and glutamate in Huntington's disease: a balancing act. CNS Neurosci Ther 16:163–178
Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK (2004) Dopamine modulates release from corticostriatal terminals. J Neurosci 24:9541–9552
David HN, Ansseau M, Abraini JH (2005) Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Rev 50:336–360
Greenamyre JT (1993) Glutamate-dopamine interactions in the basal ganglia: relationship to Parkinson's disease. J Neural Transm Gen Sect 91:255–269
Starr MS (1995) Glutamate/dopamine D1/D2 balance in the basal ganglia and its relevance to Parkinson's disease. Synapse 19:264–293
Yamamoto BK, Davy S (1992) Dopaminergic modulation of glutamate release in striatum as measured by microdialysis. J Neurochem 58:1736–1742
Greenamyre JT, Hastings TG (2004) Biomedicine. Parkinson's— divergent causes, convergent mechanisms. Science 304:1120–1122
Blandini F, Greenamyre JT, Nappi G (1996) The role of glutamate in the pathophysiology of Parkinson's disease. Funct Neurol 11:3–15
Izumi Y, Yamamoto N, Matsuo T, Wakita S, Takeuchi H, Kume T et al (2009) Vulnerability to glutamate toxicity of dopaminergic neurons is dependent on endogenous dopamine and MAPK activation. J Neurochem 110:745–755
Black YD, Xiao D, Pellegrino D, Kachroo A, Brownell AL, Schwarzschild MA (2010) Protective effect of metabotropic glutamate mGluR5 receptor elimination in 6-hydroxydopamine model of Parkinson's disease. Neurosci Lett 486:161–165
Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD et al (1998) Regulation of the glial Na + -dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol 53:355–369
Tian G, Lai L, Guo H, Lin Y, Butchbach ME, Chang Y et al (2007) Translational control of glial glutamate transporter EAAT2 expression. J Biol Chem 282:1727–1737
Dervan AG, Meshul CK, Beales M, McBean GJ, Moore C, Totterdell S et al (2004) Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson's disease. Exp Neurol 190:145–156
Wang J, Hoekstra JG, Zuo C, Cook TJ, Zhang J (2013) Biomarkers of Parkinson's disease: current status and future perspectives. Drug Discov Today 18:155–162
Bevers MB, Neumar RW (2008) Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 28:655–673
Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock JW et al (2000) Regulation of tyrosine hydroxylase activity and phosphorylation at ser19 and ser40 via activation of glutamate NMDA receptors in rat striatum. J Neurochem 74:2470–2477
Nafia I, Re DB, Masmejean F, Melon C, Kachidian P, Kerkerian-Le Goff L, Nieoullon A, Had-Aissouni L (2008) Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J Neurochem 105:484–496
Maetzler W, Liepelt I, Berg D (2009) Progression of Parkinson's disease in the clinical phase: potential markers. The Lancent Neurology 8:1158–1171
Wu Y, Le W, Jankovic J (2011) Preclinical biomarkers of Parkinson disease. Arch Neurol 68:22–30
Cenci MA, Lindgren HS (2007) Advances in under standing L-DOPA-induced dyskinesia. Current Opinion in Neurobiology 17(6):665–671
Cenci MA, Lundblad M (2006) Post- versus presynap tic plasticity in L-DOPA-induced dyskinesia. Journal of Neurochemistry 99(2):381–392
Chase TN, Oh JD (2000) Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends in Neurosciences 23(10 Suppl):S86–S91
Acknowledgments
The authors wish to thank Victoria Fields for her outstanding technical assistance at various points throughout the study. This study was funded by the Edward P. Stiles Trust Fund-LSUHSC-Shreveport and Biomedical Research Foundation of NW Louisiana awards to MFS and the Ike Muslow Predoctoral Fellowship to TC.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chotibut, T., Davis, R.W., Arnold, J.C. et al. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson's model. Mol Neurobiol 49, 1282–1292 (2014). https://doi.org/10.1007/s12035-013-8598-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8598-0