Abstract
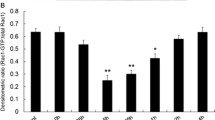
The purpose of the present study was to determine the potential for RhoA/ROCK signaling to play a role in endothelial-monocyte-activating polypeptide (EMAP) II-induced increase in blood–tumor barrier (BTB) permeability in rat brain microvascular endothelial cells (RBMECs). In the present study, we used an in vitro BTB model, a RhoA inhibitor (C3 exoenzyme) and a ROCK inhibitor (Y27632) to determine whether RhoA/ROCK pathway play a role in the process of TJ disassembly, stress fiber formation, MLC and cofilin phosphorylation, as well as increase of BTB permeability induced by EMAP II. The results revealed that BTB permeability was increased by EMAP II induction, and C3 exoenzyme or Y27632 could partially inhibit the EMAP II-induced increase of BTB permeability. The significant down-regulations in tight junction (TJ)-associated proteins occludin, claudin-5 and ZO-1 and stress fiber formation by EMAP II administration were observed, which were partly prevented by C3 exoenzyme or Y27632 pretreatment. Moreover, the significant increases in RhoA activity, myosin light chain (MLC) and cofilin phosphorylation by EMAP II administration were observed, MLC and cofilin phosphorylation were partly inhibited by C3 exoenzyme or Y27632 pretreatment. The present study demonstrates that the activation of RhoA/ROCK signaling in RBMECs was required for the increase of BTB permeability and these effects are related with the ability for RhoA/ROCK to mediate TJ disassembly and stress fiber formation by phosphorylating cofilin and MLC.









Similar content being viewed by others
References
Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, Drenckhahn D (2002) Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 539(Pt 1):295–308
Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271(34):20246–20249
Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K (1997) Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275(5304):1308–1311
Berger AC, Tang G, Alexander HR, Libutti SK (2000) Endothelial monocyte-activating polypeptide II, a tumor-derived cytokine that plays an important role in inflammation, apoptosis, and angiogenesis. J Immunother 23(5):519–527
Biegel D, Pachter JS (1994) Growth of brain microvessel endothelial cells on collagen gels: applications to the study of blood-brain barrier physiology and CNS inflammation. Vitro Cell Dev Biol Anim 30A(9):581–588
Biegel D, Spencer DD, Pachter JS (1995) Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro. Brain Res 692(1–2):183–189
Black KL, Ningaraj NS (2004) Modulation of brain tumor capillaries for enhanced drug delivery selectively to brain tumor. Cancer Control 11(3):165–173
Carbajal JM, Schaeffer RC Jr (1999) RhoA inactivation enhances endothelial barrier function. Am J Physiol 277(5 Pt 1):C955–C964
Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC Jr (2000) ROCK mediates thrombin’s endothelial barrier dysfunction. Am J Physiol Cell Physiol 279(1):C195–C204
Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA (2005) RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288(2):L294–L306
Easton AS, Abbott NJ (2002) Brandykinin increases permeability by calcium and 5-lipoxygenase in the ECV304/C6 cell culture model of the blood–brain barrier. Brain Res 953(1–2):157–169
Fuller E, Duckham C, Wood E (2007) Disruption of epithelial tight junctions by yeast enhances the paracellular delivery of a model protein. Pharm Res 24(1):37–47
Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K (2001) Molecular and cellular permeability control at the blood-brain barrier. Brain Res Rev 36(2–3):258–264
Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279(5350):509–514
Harhaj NS, Antonetti DA (2004) Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 36(7):1206–1237
Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, Yokoyama M, Staddon JM (2001) Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J Biol Chem 276(13):10423–10431
Hou Y, Plett PA, Ingram DA, Rajashekhar G, Orschell CM, Yoder MC, March KL, Clauss M (2006) Endothelial-monocyte-activating polypeptide II induces migration of endothelial progenitor cells via the chemokine receptor CXCR3. Exp Hematol 34(8):1125–1132
Hurst RD, Fritz IB (1996) Properties of an immortalised vascular endothelial/glioma cell co-culutre model of the blood-brain barrier. J Cell Physiol 167(1):81–88
Ivanov AI, McCall IC, Parkos CA, Nusrat A (2004) Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell 15(6):2639–2651
Keezer SM, Ivie SE, Krutzsch HC, Tandle A, Libutti SK, Roberts DD (2003) Angiogenesis inhibitors target the endothelial cell cytoskeleton through altered regulation of heat shock protein 27 and cofilin. Cancer Res 63(19):6405–6412
Kemper EM, Boogerd W, Thuis I, Beijnen JH, Van Tellingen O (2004) Modulation of the blood–brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev 30(5):415–423
Kniesel U, Wolburg H (2000) Tight junctions of the blood–brain barrier. Cell Mol Neurobiol 20(1):57–76
Lai CH, Kuo KH, Leo JM (2005) Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev 50(1):7–13
Lee NP, Cheng CY (2003) Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology 144(7):3114–3129
Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY (2004) Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res 68(3):231–238
Leung T, Chen XQ, Manser E, Lim L (1996) The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16(10):5313–5327
Li B, Zhao WD, Tan ZM, Fang WG, Zhu L, Chen YH (2006) Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett 580(17):4252–4260
Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, Chien S, Shyy JY (2002) Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol 22(1):76–81
Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S (1999) Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285(5429):895–898
Mark KS, Davis TP (2002) Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 282(4):H1485–H1494
McKenzie AJ, Ridley AJ (2007) Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol 213(1):221–228
Rubin LL, Staddon JM (1999) The cell biology of the blood–brain barrier. Annu Rev Neurosci 22:11–28
Salama NN, Eddington ND, Fasano A (2006) Tight junction modulation and its relationship to drug delivery. Adv Drug Deliv Rev 58(1):15–28
Scott PA, Bicknell R (1993) The isolation and culture of microvascular endothelium. J Cell Sci 105(Pt 2):269–273
Shalak V, Guigou L, Kaminska M, Wautier MP, Wautier JL, Mirande M (2007) Characterization of p43(ARF), a derivative of the p43 component of multiaminoacyl-tRNA synthetase complex released during apoptosis. J Biol Chem 282(15):10935–10943
Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV (2003) Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci 116(Pt22):4615–4628
Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH (2006) Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 13(3):237–247
Takai Y, Sasaki T, Tanaka K, Nakanishi H (1995) Rho as a regulator of the cytoskeleton. Trends Biochem Sci 20(6):227–231
Tandle AT, Mazzanti C, Alexander HR, Roberts DD, Libutti SK (2005) Endothelial monocyte activating polypeptide-II induced gene expression changes in endothelial cells. Cytokine 30(6):347–358
Tiruppathi C, Minshall RD, Malik AB (2003) RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem 278(35):33492–33500
Tsai BM, Wang M, Clauss M, Sun P, Meldrum DR (2004) Endothelial monocyte-activating polypeptide II causes NOS-dependent pulmonary artery vasodilation: a novel effect for a proinflammatory cytokine. Am J Physiol Regul Integr Comp Physiol 287(4):R767–R771
Van Aelst L, D'Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes Dev 11(18):2295–2322
Wickstrom SA, Alitalo K, Keski-Oja J (2003) Endostatin associates with lipid rafts and induces reorganization of the actin cytoskeleton via down-regulation of RhoA activity. J Biol Chem 278(39):37895–37901
Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ (2001) Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114(Pt 7):1343–1355
Wolburg H, Lippoldt A (2002) Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol 38(6):323–337
Wong D, Dorovini-Zis K, Vincent SR (2004) Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol 190(2):446–455
Xie H, Xue YX, Liu LB, Liu YH (2010) Endothelial-monocyte-activating polypeptide II increases blood-tumor barrier permeability by down-regulating the expression levels of tight junction associated proteins. Brain Res 1319:13–20
Acknowledgments
This work is supported by grants from the Natural Science Foundation of China (nos. 30670723, 30800451, 30872656, 30973079, 81001029, 81072056), the special fund for Scientific Research of Doctor-degree Subjects in Colleges and Universities, No. 20092104110015, and Shenyang Science and Technology Plan Projects (nos. F-10-205-1-22, F-10-205-1-37).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hui Xie and Yi-xue Xue contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xie, H., Xue, Yx., Liu, Lb. et al. Role of RhoA/ROCK Signaling in Endothelial-Monocyte-Activating Polypeptide II Opening of the Blood–Tumor Barrier. J Mol Neurosci 46, 666–676 (2012). https://doi.org/10.1007/s12031-011-9564-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9564-9