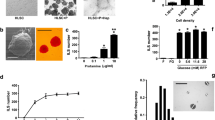
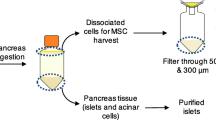
Abstract
Recent advance in directed differentiation of pancreatic stem cells offers potential to the development of replacement therapy for diabetic patients. However, the existing differentiation protocols are complex, time-consuming, and costly; thus there is a need for alternative protocols. Given the common developmental origins of liver and pancreas, we sought to develop a novel protocol, devoid of growth factors, by using liver stromal cells (LSCs) derived from human fetal liver. We examined the effects of the LSCs on the differentiation of pancreatic progenitor cells (PPCs) into islet-like cell clusters (ICCs). PPCs and LSCs isolated from 1st to 2nd trimester human fetal tissues underwent co-cultures; differentiation and functionality of ICCs were determined by examining expression of critical markers and secretion of insulin. Co-culture with 2nd but not 1st trimester LSCs enhanced ICC differentiation and functionality without the use of exogenous differentiation ‘cocktails’. Differential expression profiles of growth factors from 1st versus 2nd trimester fetal liver were compared. Many morphogenic factors were expressed by LSCs, while insulin-like growth factor 1 (IGF1) was identified as one of the key molecules responsible for the ICC differentiation. This is the first report showing that an LSC-induced microenvironment can enhance ICC differentiation and functionality. Further modifications of the stroma microenvironment may offer an alternative, efficient and cost-effective approach to providing islets for transplantation.







Similar content being viewed by others
References
Shapiro, A. M., Ricordi, C., & Hering, B. (2003). Edmonton’s islet success has indeed been replicated elsewhere. Lancet, 362, 1242.
Ryan, E. A., Paty, B. W., Senior, P. A., et al. (2005). Five-year follow-up after clinical islet transplantation. Diabetes, 54, 2060–2069.
de Kort, H., de Koning, E. J., Rabelink, T. J., Bruijn, J. A., & Bajema, I. M. (2011). Islet transplantation in type 1 diabetes. BMJ, 342, d217.
Leung, P. S., & Ng, K. Y. (2013). Current progress in stem cell research and its potential for islet cell transplantation. Current Molecular Medicine, 13, 109–125.
Kordowich, S., Mansouri, A., & Collombat, P. (2010). Reprogramming into pancreatic endocrine cells based on developmental cues. Molecular and Cellular Endocrinology, 323, 62–69.
Juhl, K., Bonner-Weir, S., & Sharma, A. (2010). Regenerating pancreatic beta-cells: plasticity of adult pancreatic cells and the feasibility of in-vivo neogenesis. Current Opinion in Organ Transplantation, 15, 79–85.
Suen, P. M., Zou, C., Zhang, Y. A., et al. (2008). PDZ-domain containing-2 (PDZD2) is a novel factor that affects the growth and differentiation of human fetal pancreatic progenitor cells. International Journal of Biochemistry and Cell Biology, 40, 789–803.
Leung, K. K., Suen, P. M., Lau, T. K., Ko, W. H., Yao, K. M., & Leung, P. S. (2009). PDZ-domain containing-2 (PDZD2) drives the maturity of human fetal pancreatic progenitor-derived islet-like cell clusters with functional responsiveness against membrane depolarization. Stem Cells and Development, 18, 979–990.
Ma, M. T., Leung, K. K., Tsang, K. S., & Leung, P. S. (2011). Reduced immunogenicity of pancreatic progenitor cells derived from first-trimester human fetal pancreas. International Journal of Biochemistry and Cell Biology, 43, 812–820.
Leung, K. K., Liang, J., Ma, M. T., & Leung, P. S. (2012). Angiotensin II type 2 receptor is critical for the development of human fetal pancreatic progenitor cells into islet-like cell clusters and their potential for transplantation. Stem Cells, 30, 525–536.
Ng, K. Y., Ma, M. T., Leung, K. K., & Leung, P. S. (2011). Vitamin D and vitamin A receptor expression and the proliferative effects of ligand activation of these receptors on the development of pancreatic progenitor cells derived from human fetal pancreas. Stem Cell Reviews, 7, 53–63.
Krebsbach, P. H., Kuznetsov, S. A., Bianco, P., & Robey, P. G. (1999). Bone marrow stromal cells: characterization and clinical application. Critical Reviews in Oral Biology and Medicine, 10, 165–181.
Alphonso, A., & Alahari, S. K. (2009). Stromal cells and integrins: conforming to the needs of the tumor microenvironment. Neoplasia, 11, 1264–1271.
Soto-Gutierrez, A., Navarro-Alvarez, N., Caballero-Corbalan, J., Tanaka, N., & Kobayashi, N. (2008). Endoderm induction for hepatic and pancreatic differentiation of ES cells. Acta Medica Okayama, 62, 63–68.
Lee, K. Y., Fong, B. S., Tsang, K. S., et al. (2011). Fetal stromal niches enhance human embryonic stem cell-derived hematopoietic differentiation and globin switch. Stem Cells and Development, 20, 31–38.
Luo, L., Badiavas, E., Luo, J. Z., & Maizel, A. (2007). Allogeneic bone marrow supports human islet beta cell survival and function over 6 months. Biochemical and Biophysical Research Communications, 361, 859–864.
Sordi, V., Melzi, R., Mercalli, A., et al. (2010). Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells, 28, 140–151.
Deutsch, G., Jung, J., Zheng, M., Lora, J., & Zaret, K. S. (2001). A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development, 128, 871–881.
Zaret, K. S., & Grompe, M. (2008). Generation and regeneration of cells of the liver and pancreas. Science, 322, 1490–1494.
Si-Tayeb, K., Lemaigre, F. P., & Duncan, S. A. (2010). Organogenesis and development of the liver. Developmental Cell, 18, 175–189.
Imai, J., Katagiri, H., Yamada, T., et al. (2008). Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science, 322, 1250–1254.
Starzl, T. E., Jones, A. F., Terblanche, J., Usui, S., Porter, K. A., & Mazzoni, G. (1979). Growth-stimulating factor in regenerating canine liver. Lancet, 1(8108), 127–130.
Adams, G. A., Maestri, M., Squiers, E. C., Alfrey, E. J., Starzl, T. E., & Dafoe, D. C. (1998). Augmenter of liver regeneration enhances the success rate of fetal pancreas transplantation in rodents. Transplantation, 65, 32–36.
Le Roith, D. (1997). Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. New England Journal of Medicine, 336, 633–640.
Baker, J., Liu, J. P., Robertson, E. J., & Efstratiadis, A. (1993). Role of insulin-like growth factors in embryonic and postnatal growth. Cell, 75, 73–82.
Lingohr, M. K., Dickson, L. M., McCuaig, J. F., Hugl, S. R., Twardzik, D. R., & Rhodes, C. J. (2002). Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-alpha or EGF, augments pancreatic beta-cell proliferation. Diabetes, 51, 966–976.
Hugl, S. R., White, M. F., & Rhodes, C. J. (1998). Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. Journal of Biological Chemistry, 273, 17771–17779.
Withers, D. J., Burks, D. J., Towery, H. H., Altamuro, S. L., Flint, C. L., & White, M. F. (1999). Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nature Genetics, 23, 32–40.
George, M., Ayuso, E., Casellas, A., Costa, C., Devedjian, J. C., & Bosch, F. (2002). Beta cell expression of IGF-I leads to recovery from type 1 diabetes. Journal of Clinical Investigation, 109, 1153–1163.
Agudo, J., Ayuso, E., Jimenez, V., et al. (2008). IGF-I mediates regeneration of endocrine pancreas by increasing beta cell replication through cell cycle protein modulation in mice. Diabetologia, 51, 1862–1872.
Suen, P. M., Li, K., Chan, J. C., & Leung, P. S. (2006). In vivo treatment with glucagon-like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. International Journal of Biochemistry and Cell Biology, 38, 951–960.
Sneddon, J. B., Borowiak, M., & Melton, D. A. (2012). Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature, 491, 765–768.
Sarkar, S. A., Kobberup, S., Wong, R., et al. (2008). Global gene expression profiling and histochemical analysis of the developing human fetal pancreas. Diabetologia, 51, 285–297.
Joglekar, M. V., Joglekar, V. M., Joglekar, S. V., & Hardikar, A. A. (2009). Human fetal pancreatic insulin-producing cells proliferate in vitro. Journal of Endocrinology, 201, 27–36.
Zhang, L., Theise, N., Chua, M., & Reid, L. M. (2008). The stem cell niche of human livers: symmetry between development and regeneration. Hepatology, 48, 1598–1607.
Tanimizu, N., Tsujimura, T., Takahide, K., Kodama, T., Nakamura, K., & Miyajima, A. (2004). Expression of Dlk/Pref-1 defines a subpopulation in the oval cell compartment of rat liver. Gene Expression Patterns, 5, 209–218.
Mitchell, J. B., McIntosh, K., Zvonic, S., et al. (2006). Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells, 24, 376–385.
Jensen, C. H., Jauho, E. I., Santoni-Rugiu, E., et al. (2004). Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. American Journal of Pathology, 164, 1347–1359.
Ledran, M. H., Krassowska, A., Armstrong, L., et al. (2008). Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell, 3, 85–98.
Campagnoli, C., Fisk, N., Overton, T., Bennett, P., Watts, T., & Roberts, I. (2000). Circulating hematopoietic progenitor cells in first trimester fetal blood. Blood, 95, 1967–1972.
Terrace, J. D., Hay, D. C., Samuel, K., et al. (2009). Side population cells in developing human liver are primarily haematopoietic progenitor cells. Experimental Cell Research, 315, 2141–2153.
Segev, H., Fishman, B., Ziskind, A., Shulman, M., & Itskovitz-Eldor, J. (2004). Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells, 22, 265–274.
Brolen, G. K., Heins, N., Edsbagge, J., & Semb, H. (2005). Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes, 54, 2867–2874.
D’Amour, K. A., Bang, A. G., Eliazer, S., et al. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnology, 24, 1392–1401.
Ranjan, A. K., Joglekar, M. V., & Hardikar, A. A. (2009). Endothelial cells in pancreatic islet development and function. Islets, 1, 2–9.
Lammert, E., Cleaver, O., & Melton, D. (2001). Induction of pancreatic differentiation by signals from blood vessels. Science, 294, 564–567.
Kanaka-Gantenbein, C., Dicou, E., Czernichow, P., & Scharfmann, R. (1995). Presence of nerve growth factor and its receptors in an in vitro model of islet cell development: implication in normal islet morphogenesis. Endocrinology, 136, 3154–3162.
Movassat, J., Beattie, G. M., Lopez, A. D., Portha, B., & Hayek, A. (2003). Keratinocyte growth factor and beta-cell differentiation in human fetal pancreatic endocrine precursor cells. Diabetologia, 46, 822–829.
Li, J., Goodyer, C. G., Fellows, F., & Wang, R. (2006). Stem cell factor/c-Kit interactions regulate human islet-epithelial cluster proliferation and differentiation. International Journal of Biochemistry and Cell Biology, 38, 961–972.
Ameri, J., Stahlberg, A., Pedersen, J., et al. (2010). FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells, 28, 45–56.
Kido, Y., Nakae, J., Hribal, M. L., Xuan, S., Efstratiadis, A., & Accili, D. (2002). Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. Journal of Biological Chemistry, 277, 36740–36747.
Kulkarni, R. N., Holzenberger, M., Shih, D. Q., et al. (2002). Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nature Genetics, 31, 111–115.
Smith, F. E., Rosen, K. M., Villa-Komaroff, L., Weir, G. C., & Bonner-Weir, S. (1991). Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proceedings of the National Academy of Sciences of the United States of America, 88, 6152–6156.
Wang, R. N., Kloppel, G., & Bouwens, L. (1995). Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia, 38, 1405–1411.
Warzecha, Z., Dembinski, A., Ceranowicz, P., et al. (2003). IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. Journal of Physiology and Pharmacology, 54, 575–590.
Kulkarni, R. N. (2005). New insights into the roles of insulin/IGF-I in the development and maintenance of beta-cell mass. Reviews in Endocrine & Metabolic Disorders, 6, 199–210.
Acknowledgments
The work described in this paper was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project no. 468912), and from the School of Biomedical Sciences Group Research Grant, The Chinese University of Hong Kong (Project no. SBS-SEED-PSL), awarded to PS Leung.
Competing Financial Interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Juan Liang and Ka Yan Ng contributed equally to this paper
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
Primers sequences used for mRNA expression studies (DOC 76 kb)
Supplemental Table 2
Information of primary antibodies used in this study (DOC 34 kb)
Rights and permissions
About this article
Cite this article
Liang, J., Ng, K.Y., Cheng, Q. et al. Human Fetal Liver Stromal Cell Co-Culture Enhances the Differentiation of Pancreatic Progenitor Cells into Islet-Like Cell Clusters. Stem Cell Rev and Rep 10, 280–294 (2014). https://doi.org/10.1007/s12015-013-9491-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-013-9491-y