Abstract
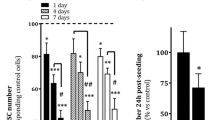
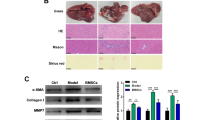
Transplantation of bone marrow (BM)-derived endothelial progenitor cells (EPCs) has been reported to improve liver fibrosis, but there is no direct evidence for the mechanism of improvement. We investigated the mechanism in vitro by coculturing BM-derived EPCs with activated hepatic stellate cells (HSCs) to mimic the hepatic environment. EPCs and HSCs were cultured alone and indirectly cocultured at a 1:1 ratio in a Transwell system. The characteristics of HSCs and EPCs were examined at different time points. An invasion assay showed the time-dependent effect on degradation of the extracellular matrix (ECM) layer in EPCs cultured alone. Real-time PCR and enzyme-linked immunosorbent assay analysis revealed that EPCs served as a source of matrix metalloproteinase-9 (MMP-9), and MMP-9 expression levels significantly increased during the 2 d of coculture. CFSE labeling showed that EPCs inhibited proliferation of HSCs. Annexin-V/PI staining, erminal deoxynucleotidyl transferase X-dUTP nick end labeling analysis, and (cleaved) caspase-3 activity revealed that EPCs promoted HSC apoptosis. However, the proliferation and apoptosis of EPCs were unaffected by cocultured HSCs. Coculturing increased the expression of inducible nitric oxide synthase, vascular endothelial growth factor, and hepatocyte growth factor (HGF) in EPCs, promoted differentiation of EPCs, and reduced the expression of types I and III collagens and transforming growth factor beta 1. Knockdown of HGF expression attenuated EPC-induced activation of HSC apoptosis and profibrotic ability. These findings demonstrated that BM-derived EPCs could degrade ECM, promoting activated HSC apoptosis, suppressing proliferation and profibrotic ability of activated HSCs. HGF secretion by EPCs plays a key role in inducing activated HSC apoptosis and HSC profibrotic ability.









Similar content being viewed by others
References
Deleve L. D.; Wang X.; Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48: 920–930; 2008.
Fausto N. Liver regeneration. J Hepatol 32(1 Suppl): 19–31; 2000.
Friedman S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology 134: 1655–1669; 2008.
Fürst G.; Schulte Am Esch J.; Poll L. W.; Hosch S. B.; Fritz L. B.; Klein M.; Godehardt E.; Krieg A.; Wecker B.; Stoldt V.; Stockschläder M.; Eisenberger C. F.; Mödder U.; Knoefel W. T. Portal vein embolisation and autologous CD133+ bone marrow stem cells for liver regeneration: initial experience. Radiology 243: 171–179; 2007.
Gaia S.; Smedile A.; Omedè P.; Olivero A.; Sanavio F.; Balzola F.; Ottobrelli A.; Abate M. L.; Marzano A.; Rizzetto M.; Tarella C. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol 45: 13–19; 2006.
Han Y. P. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol 21(Suppl 3): S88–S91; 2006.
Henderson N. C.; Forbes S. J. Hepatic fibrogenesis: from within and outwith. Toxicology 254: 130–135; 2008.
Ii M.; Nishimura H.; Iwakura A.; Wecker A.; Eaton E.; Asahara T.; Losordo D. W. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 111: 1114–1120; 2005.
Kurschat P.; Zigrino P.; Nischt R.; Breitkopf K.; Steurer P.; Klein C. E.; Krieg T.; Mauch C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J Biol Chem 274: 21056–21062; 1999.
Langer D. A.; Das A.; Semela D.; Kang-Decker N.; Hendrickson H.; Bronk S. F.; Katusic Z. S.; Gores G. J.; Shah V. H. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology 47: 1983–1993; 2008.
Li J. T.; Liao Z. X.; Ping J.; Xu D.; Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol 43: 419–428; 2008.
Liu F.; Liu Z. D.; Wu N.; Cong X.; Fei R.; Chen H. S.; Wei L. Transplanted endothelial progenitor cells ameliorate carbon tetrachloride-induced liver cirrhosis in rats. Liver Transpl 15: 1092–1100; 2009.
Mohamadnejad M.; Alimoghaddam K.; Mohyeddin-Bonab M.; Bagheri M.; Bashtar M.; Ghanaati H.; Baharvand H.; Ghavamzadeh A.; Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 10: 459–466; 2007.
Nakamura T.; Torimura T.; Iwamoto H.; Masuda H.; Naitou M.; Koga H.; Abe M.; Hashimoto O.; Tsutsumi V.; Ueno T.; Sata M. Prevention of liver fibrosis and liver reconstitution of DMN-treated rat liver by transplanted EPCs. Eur J Clin Invest 42: 717–728; 2012.
Nakamura T.; Torimura T.; Sakamoto M.; Hashimoto O.; Taniguchi E.; Inoue K.; Sakata R.; Kumashiro R.; Murohara T.; Ueno T.; Sata M. Significance and therapeutic potential of endothelial progenitor cell transplantation in a cirrhotic liver rat model. Gastroenterology 133: e1–107; 2007.
Reynaert H.; Chavez M.; Geerts A. Vascular endothelial growth factor and liver regeneration. J Hepatol 34: 759–761; 2001.
Schächinger V.; Assmus B.; Britten M. B.; Honold J.; Lehmann R.; Teupe C.; Abolmaali N. D.; Vogl T. J.; Hofmann W. K.; Martin H.; Dimmeler S.; Zeiher A. M. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 106: 3009–3017; 2007.
Terai S.; Ishikawa T.; Omori K.; Aoyama K.; Marumoto Y.; Urata Y.; Yokoyama Y.; Uchida K.; Yamasaki T.; Fujii Y.; Okita K.; Sakaida I. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 24: 2292–2298; 2006.
Ueki T.; Kaneda Y.; Tsutsui H.; Nakanishi K.; Sawa Y.; Morishita R.; Matsumoto K.; Nakamura T.; Takahashi H.; Okamoto E.; Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 5: 226–230; 1999.
Yannaki E.; Anagnostopoulos A.; Kapetanos D.; Xagorari A.; Iordanidis F.; Batsis I.; Kaloyannidis P.; Athanasiou E.; Dourvas G.; Kitis G.; Fassas A. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol 34: 1583–1587; 2006.
Acknowledgements
This work was supported by the National Science Foundation Fund of China (No. 30700350), the Major State Basic Research Development Program of China (973) (No. 2005CB522902 and 2007CB512900), and Peking University People’s Hospital Research and Development Funds (No. RDK2008-06) and sponsored by a National Science and Technology Major Project (2012ZX10002003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Feng Liu and Zhi-da Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, F., Liu, Zd., Wu, N. et al. In vitro interactions between rat bone marrow-derived endothelial progenitor cells and hepatic stellate cells. In Vitro Cell.Dev.Biol.-Animal 49, 537–547 (2013). https://doi.org/10.1007/s11626-013-9637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-013-9637-x