Summary
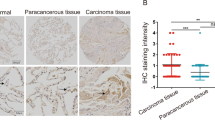
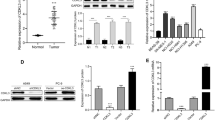
This study examined the role of EMP-1 in tumorigenesis of non-small cell lung carcinoma (NSCLC) and the possible mechanism. Specimens were collected from 28 patients with benign lung diseases and 28 with NSCLC, and immunohistochemically detected to evaluate the correlation of EMP-1 expression to the clinical features of NSCLC. Recombinant adenovirus was constructed to over-express EMP-1 and then infect PC9 cells. Cell proliferation was measured by Ki67 staining. Western blotting was performed to examine the effect of EMP-1 on the PI3K/AKT signaling. Moreover, tumor xenografts were established by subcutaneous injection of PC9 cell suspension (about 5×107/mL in 100 μL of PBS) into the right hind limbs of athymic nude mice. The results showed EMP-1 was significantly up-regulated in NSCLC patients as compared with those with benign lung diseases. Over-expression of EMP-1 promoted proliferation of PC9 cells, which coincided with the activation of the PI3K/AKT pathway. EMP-1 promoted the growth of xenografts of PC9 cells in athymic nude mice. It was concluded that EMP-1 expression may contribute to the development and progress of NSCLC by activating PI3K/AKT pathway.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin, 2008,58(2):71–96
Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science, 2004,304(5676):1497–1500
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med, 2004,350(21):2129–2139
Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA, 2004,101(36):13306–13311
Eberhard DA, Giaccone G, Johnson BE. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol, 2008,26(6):983–994
Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev, 2008,18(1):73–79
Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med, 2005,2(1):e17
Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med, 2005,352(8):786–792
Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res, 2006,12(21):6494–6501
Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res, 2006,12(19):5764–5769
Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science, 2007,316(5827): 1039–1043
Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA, 2007,104(52):20932–20937
Marvin KW, Fujimoto W, Jetten AM. Identification and characterization of a novel squamous cell-associated gene related to PMP22. J Biol Chem, 1995,270(48):28910–28916
Schiemann S, Valentine M, Weidle UH. Assignment of the human progression associated protein (PAP) to chromosome 12p12.3. Anticancer Res, 1997,17(6D):4281–4285
Ben-Porath I, Benvenisty N. Characterization of a tumour-associated gene, a member of a novel family of genes encoding membrane glycoproteins. Gene, 1996,183(1–2):69–75
Ruegg CL, Wu HY, Fagnoni FF, et al. B4B, a novel growth-arrest gene, is expressed by a subset of progenitor/pre-B lymphocytes negative for cytoplasmic mu-chain. J Immunol, 1996,157(1):72–80
Taylor V, Welcher AA, Program AE, et al. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J Biol Chem, 1995,270(48):28824–28833
Ahn N, Kim S, Choi W, et al. Extracellular matrix protein gene, EMP1, is required for appressorium formation and pathogenicity of the rice blast fungus, Magnaporthe grisea. Mol Cells, 2004,17(1):166–173
Jain A, Tindell CA, Laux I, et al. Epithelial membrane protein-1 is a biomarker of gefitinib resistance. Proc Natl Acad Sci USA, 2005,102(33):11858–11863
Yu YH, Kuo HK, Chang KW, et al. The evolving transcriptome of head and neck squamous cell carcinoma: A systematic review. PLoS ONE, 2008,3(9):e3215
Johnson AH, Frierson HF, Zaika A, et al. Expression of tight-junction protein claudin-7 is an early event in gastric tumorigenesis. Am J Pathol, 2005,167(2):577–584
Turashvili G, Bouchal J, Baumforth K, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer, 2007,7:55
He TC, Zhou S, da Costa LT, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA, 1998,95(5):2509–2514
Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature Rev Drug Discov, 2009,8(8):627–644.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet, 2006,7(8):606–619
Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008,27(41), 5497–5510
Alessi DR, Deak M, Casamayor A, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol, 1997,7(10):776–789
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell, 2007,129(7):1261–1274
Samuels, Y, Diaz LA Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell, 2005,7(6):561–573
Carpten, JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature, 2007,448(7152):439–444
Stephens L, Anderson K, Stokoe D, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science, 1998, 279,(5351):710–714
Zhang J, Xu Q, Chen WT. The expression of EMP1 is downregulated in oral squamous cell carcinoma and possibly associated with tumour metastasis. J Clin Patho, 2011,64(1):25–29
Li YQ, Xue T, Wang L, et al. Up-regulation of epithelial membrane protein-1 in the temporal neocortex of patients with intractable epilepsy. Neurochem Res, 2009,34(9): 1594–1602
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from the National Natural Science Foundation of China (Nos. 81072431, 30872472, 30973496 and 30800569), the Innovative Foundation of Huazhong University of Science and Technology (No. 2010MS027), and the Foundation of “973” Program (No. 2009CB521802), and by Special Fund for Central University Basic Scientific Research (Nos. 2011JC062, 2011JC063).
Rights and permissions
About this article
Cite this article
Lai, S., Wang, G., Cao, X. et al. EMP-1 promotes tumorigenesis of NSCLC through PI3K/AKT pathway. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 834–838 (2012). https://doi.org/10.1007/s11596-012-1043-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-1043-1