Summary
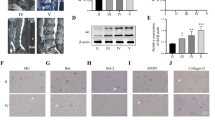
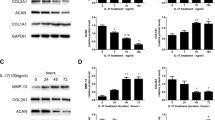
The present study examined the role of Wnt/β-catenin signaling pathway in the degeneration of nucleus pulposus cells and the protective effect of DKK1 on nucleus pulposus cells. The model of nucleus pulposus cell degeneration was induced by intra-disc injection of TNF-α, and the expression of β-catenin protein was detected by Western blotting. The cultured rabbit nucleus pulposus cells were divided into 4 groups. In group A, the cells were cultured with normal medium and served as control group. In group B, the cells were cultured with TNF-α and acted as degeneration group. In group C, the cells were cultured with TNF-α and transfected with Adv-eGFP and was used as fluorescence control group. In group D, the cells were cultured with TNF-α and transfected with Adv-hDKK1-eGFP, serving as intervention group. The expression of type II collagen, proteoglycan, β-catenin, and MMP-13 in each group was detected by immunocytochemistry and RT-PCR. The result showed that TNF-α increased the expression of β-catenin and MMP-13, and significantly inhibited the synthesis of type II collagen and proteoglycan, which resulted in the degeneration of nucleus pulposus cells. This effect could be obviously reversed by DKK1. We are led to concluded that TNF-α could activate the Wnt/β-catenin signaling pathway, and increase the expression of MMP-13, thereby resulting in disc degeneration. Specifically blocking Wnt/β-catenin signaling pathway by DKK-1 could protect the normal metabolism of intervertebral disc tissue. The Wnt pathway plays an important role in the progression of the intervertebral disc degeneration.
Similar content being viewed by others
References
Ryu JH, Chun JS. Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem, 2006,281(31):22039–22047
Chen Y, Whetstone HC, Youn A, et al. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem, 2007,282(1):526–533
Kluba T, Niemeyer T, Gaissmaier C, et al. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine, 2005,30(24):2743–2748
Svanvik T, Henriksson HB, Karlsson C, et al. Human disk cells from degenerated disks and mesenchymal stem cells in co-culture result in increased matrix production. Cells Tissues Organs, 2010,191(1):2–11
Loreto C, Musumeci G, Castorina A, et al. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin-positive cells and cell death. Ann Anat, 2011,193(2):156–162
Weiler C, Nerlich AG, Bachmeier BE, et al. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine, 2005,30(1):44–53
Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford), 2008,47(6): 809–814
Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem, 2006,281(14):9507–9516
Rutges J, Creemers LB, Dhert W, et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage, 2010,18(3):416–423
Clouet J, Grimandi G, Pot-Vaucel M, et al. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford), 2009,48(11):1447–1450
Chan BY, Fuller ES, Russell AK, et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage, 2011,19(7): 874–885
Weng LH, Wang CJ, Ko JY, et al. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum, 2010,62(5):1393–1402
Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol, 2004,204(1):47–54
Yuasa T, Otani T, Koike T, et al. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest, 2008,88(3):264–274
Hiyama A, Sakai D, Risbud MV, et al. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum, 2010,62(10):3036–3047
Zhu M, Tang D, Wu Q, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res, 2009,24(1):12–21
Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem, 2005,280(19): 19185–19195
Dai J, Hall CL, Escara-Wilke J, et al. Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res, 2008,68(14):5785–5794
Aguilera O, Fraga MF, Ballestar E, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene, 2006,25(29): 4116–4121
González-Sancho JM, Aguilera O, García JM, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene, 2005,24(6):1098–1103
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, S., Wang, J., Yang, S. et al. Specific inhibitory protein Dkk-1 blocking Wnt/β-catenin signaling pathway improve protectives effect on the extracellular matrix. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 31, 657–662 (2011). https://doi.org/10.1007/s11596-011-0577-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-011-0577-y