Summary
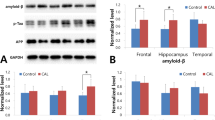
The changes in the tau protein phosphorylation and expression of bcl-2, and bax in rat parietal cortex neurons after focal cerebral ischemia-reperfusion (I/R) were explored, and the relationship between the tau protein phosphorylation and the expression of bax or apoptosis was clarified in order to elucidate the relationship between cerebral infarction and Alzheimer’s disease. The rat focal cerebral I/R model was induced by occlusion of the right middle cerebral artery using the intraluminal suture method. The level of tau protein phosphorylation at Ser396, Ser404, Tyr231, Ser199/202 sites and the expression of bcl-2, bax and total tau 5 in rat parietal cortex during focal cerebral ischemia/reperfusion were detected by Western blot. The relationship between the tau protein phosphorylation and the expression of bax, or apoptosis was examined by TUNEL method and double-labeling immunofluorenscence method. The results showed that the level of tau hyperphosphorylation at Ser199 / 202, Ser396, Ser404, Tyr231 sites and the expression levels of bcl-2, and bax were significantly higher in I/R group than in the sham group, but the ratio of bcl-2/bax was decreased. Neuronal apoptosis, bax expression and the tau protein hyperphosphorylation were co-localized. It is suggested that Alzheimer’s disease-like pathological changes occur after cerebral I/R. The highly abnormal phosphorylation of tau protein plays a key role in cerebral I/R-induced apoptosis. The cerebral infarction may contribute to Alzheimer’s disease occurrence and development.
Similar content being viewed by others
References
Kalaria R. Similarities between Alzheimer’s disease and vascular dementia. J Neurol Sci, 2002,203–205(29–34):29–34
Tatemichi TK, Desmond DW, Mayeux R, et al. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology, 1992,42(6):1185–1193
Tatemichi TK, Paik M, Bagiella E, et al. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology, 1994,44(10):1885–1891
De la Torre JC. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol, 2004,3(3):184
Götz J, Chen F, van Dorpe J, et al. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science, 2001,293(5534):1446–1447
Kobayashi K, Nakano H, Hayashi M, et al. Association of phosphorylation site of tau protein with neuronal apoptosis in Alzheimer’s disease. J Neurol Sci, 2003,208(1–2):17–24
MacGibbon GA, Lawlor PA, Sirimanne ES, et al. Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res, 1997,750(1–2):223–234
Tortosa A, Lopea E, Ferrer I. Bcl- 2 and Bax protein expression in Alzheimer’s disease. Acta Neuropathol, 1998,95(4):407–412
Zhao H, Yenari MA, Cheng D, et al. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem, 2003,85(4):1026–1036
Zhu Y, Prehn J, Clumsee C, et al. The beta-2-adrnoceptor agonist clenbuterol modulates Bcl-2, Bcl-xl and Bax protein expression following transient forebrain ischemia. Neuroscience, 1999,90(4):1255–1263
Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke, 1989,20(1):84–91
Shawn NW, Vladimir CH, David FC, et al. Interaction between a rat model of cerebral ischemia and β- amyloid toxicity. Inflammatory Responses, Stroke, 2005,36(1):107–112
Li J, Wang YJ, Zhang M, et al. Effect of cerebral ischemia on the expression of abnormally phosphorylated tau protein in cortical and hippocampal neurons of AD rats. Chongqing Med (Chinese), 2008,37(7):678–682
Ehehalt R, Keller P, Haass C, et al. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol, 2003,160(1):113–123
Honig LS, Kukull W, Mayeux R, et al. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology, 2005,64(3):494–500
Bellew KM, Pigeon JG, Stang PE, et al. Hypertension and the rate of cognitive decline in patients with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord, 2004,18(4):208–213
Kopke E, Tung YC, Shaikh S, et al. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem, 1993, 268(32):24 374–24 384
Cardona-Gómez GP, Arango-Davila C, Gallego-Gómez JC, et al. Estrogen dissociates Tau and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor subunit in postischemic hippocampus. Neuroreport, 2006,17(12):1337–1341
Ikeda K, Akiyama H, Arai T, et al. Neurons containing Alz-50-immunoreactive granules around the cerebral infarction: evidence for the lysosomal degradation of altered tau in human brain? Neurosci Lett, 2000,284(3):187–189
Wen Y, Yang SH, Liu R, et al. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Research, 2004,1022(1–2):30–38
Gong CX, Wegiel J, Lidsky T, et al. Regulation of phosphorylation of neuronal microtubule-associated proteins MAP1b and MAP2 by protein phosphatase-2A and -2B in rat brain. Brain Res, 2000,853(2):299–309
Roth KA. Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol, 2001,60(9):829–838
Katsuji K, Hiroyuki N, Masahiro H, et al. Association of phosphorylation site of tau protein with neuronal apoptosis in Alzheimer’s disease. J Neurol Sci, 2003, 208(1–2):17–24
MacGibbon GA, Lawlor PA, Sirimanne ES, et al. Bax expression in mammalian neurons undergoing apoptosis, and in Alzheimer’s disease hippocampus. Brain Res, 1997,750(1):223–234
Guise S, Braguer D, Carles G, et al. Hyperphosphorylation of tau is mediated by ERK activation during anticancer drug-induced apoptosis in neuroblstoma cells. Neurosci Res, 2001,63(3):257–267
Tortosa A, Lopea E, Ferrer I, et al. Bcl- 2 and Bax protein expression in Alzheimer’s disease. Acta Neuropathol, 1998,95(4):407–412
Chen LQ, Wei JS, Lei ZN, et al. Induction of Bcl-2 and Bax was related to hyperphosphorylation of Tau and neuronal death induced by okadaic acid in rat brain. Anat Rec A Discov Mol Cell Evol Biol, 2005,287A(2):1236–1245
Eberspacher E, Werner C, Engelhard K, et al. The effect of hypothermia on the expression of the apoptosis-regulating protein Bax after incomplete cerebral ischemia and reperfusion in rats, J Neurosurgy Anesthesiol, 2003,15(3):200–208
Amemiya S, Kamiya T, Nito C, et al. Anti-apoptotic and neuro-protective effect of edaiavone following transient focal ischemia in rats. Eur J Pharmacol, 2005,516(2):125–130
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from the National Science & Technology Pillar Program in the Eleventh Five-year Period in China (No. 2006BAI01A13).
Rights and permissions
About this article
Cite this article
Wang, H., Zhao, H., Ye, Y. et al. Focal cerebral ischemia induces Alzheimer’s disease-like pathological change in rats. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 30, 29–36 (2010). https://doi.org/10.1007/s11596-010-0106-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-010-0106-4