Abstract
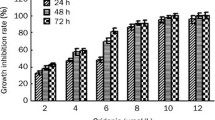
This study aimed to investigate the effects of arsenic trioxide (As2O3) on the mitochondrial DNA (mtDNA) of acute promyelocytic leukemia (APL) cells. The NB4 cell line was treated with 2.0 μmol/L As2O3in vitro, and the primary APL cells were treated with 2.0 μmol/L As2O3in vitro and 0.16 mg kg−1 d−1 As2O3in vivo. The mitochondrial DNA of all the cells above was amplified by PCR, directly sequenced and analyzed by Sequence Navigatore and Factura software. The apoptosis rates were assayed by flow cytometry. Mitochondrial DNA mutation in the D-loop region was found in NB4 and APL cells before As2O3 use, but the mutation spots were remarkably increased after As2O3 treatment, which was positively correlated to the rates of cellular apoptosis, the correlation coefficient: rNB4-As2O3=0.973818, and rAPL-As2O3=0.934703. The mutation types include transition, transversion, codon insertion or deletion, and the mutation spots in all samples were not constant and regular. It is revealed that As2O3 aggravates mtDNA mutation in the D-loop region of acute promyelocytic leukemia cells both in vitro and in vivo. Mitochondrial DNA might be one of the targets of As2O3 in APL treatment.
Similar content being viewed by others
References
Liu J, Lu Y, Wu Q, et al. Mineral arsenicals in traditional medicines: Orpiment, realgar, and arsenolite. J Pharmacol Exp Ther, 2008, 326: 363–368, 10.1124/jpet.108.139543, 1:CAS:528:DC%2BD1cXpsVWru7o%3D, 18463319
Chou W C, Chen H Y, Yu S L, et al. Arsenic suppresses gene expression in promyelocytic leukemia cells partly through Sp1 oxidation. Blood, 2005, 106: 304–310, 10.1182/blood-2005-01-0241, 1:CAS:528:DC%2BD2MXlvVWjtrg%3D, 15761015
Zhou P, Kalakonda N, Comenzo R L. Changes in gene expression profiles of multiple myeloma cells induced by arsenic trioxide (ATO): Possible mechanisms to explain ATO resistance in vivo. Br J Haematol, 2005, 128: 636–644, 10.1111/j.1365-2141.2005.05369.x, 1:CAS:528:DC%2BD2MXjtVGqsrc%3D, 15725085
Park W H, Seol J G, Kim E S, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res, 2000, 60: 3065–3071, 1:CAS:528:DC%2BD3cXjvFOltLo%3D, 10850458
Yim E K, Tong S Y, Ho E M, et al. Anticancer effects on TACC3 by treatment of paclitaxel in HPV-18 positive cervical carcinoma cells. Oncol Rep, 2009, 21: 549–557, 1:CAS:528:DC%2BD1MXit1GrtL4%3D, 19148534
Yoon P, Giafis N, Smith J, et al. Activation of mammalian target of rapamycin and the p70 S6 kinase by arsenic trioxide in BCR-ABL-expressing cells. Mol Cancer Ther, 2006, 5: 2815–2823, 10.1158/1535-7163.MCT-06-0263, 1:CAS:528:DC%2BD28Xht1els7bL, 17121928
Patlolla A K, Tchounwou P B. Cytogenetic evaluation of arsenic trioxide toxicity in Sprague-Dawley rats. Mutat Res. 2005, 587: 126–133, 1:CAS:528:DC%2BD2MXhtFKmsrfI, 16213187
Rigoli L, DiBella C, Verginelli F, et al. Histological heterogeneity and somatic mtDNA mutations in gastric intraepithelial neoplasia. Mod Pathol, 2008, 21: 733–741, 10.1038/modpathol.2008.58, 1:CAS:528:DC%2BD1cXmtFGntLo%3D, 18425082
Luciane R C, Bertrand C L. Mutagenesis, tumorigenicity and apoptosis: Are the mitochondria involved? Mutat Res, 1998, 398: 19–26
Singh K K, Kulawiec M. Mitochondrial DNA polymorphism and risk of cancer. Methods Mol Biol, 2009, 471: 291–303, 10.1007/978-1-59745-416-2_15, 1:CAS:528:DC%2BD1cXhsVGgur7L, 19109786
Mayr J A, Meierhofer D, Zimmermann F, et al. Loss of complex I due to mitochondrial DNA mutations in renal oncocytoma. Clin Cancer Res, 2008, 14: 2270–2275, 10.1158/1078-0432.CCR-07-4131, 1:CAS:528:DC%2BD1cXkvFGmt7Y%3D, 18413815
Nirajan B G, Bhat N K, Avadhani N G. Preferential attack of mitochondrial DNA by aflatoxin B1 during hepatocarcinogenesis. Science, 1982, 215: 73–75, 10.1126/science.6797067
Dasgupta S, Hoque M O, Upadhyay S, et al. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res, 2008, 68: 700–706, 10.1158/0008-5472.CAN-07-5532, 1:CAS:528:DC%2BD1cXhtlygs7w%3D, 18245469
Backer J M, Weinstein I B. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzopyrene. Science, 1980, 209: 297–299, 10.1126/science.6770466, 1:CAS:528:DyaL3cXlt1Kku7g%3D, 6770466
Wheelhouse N M, Lai P B, Wigmore S J, et al. Mitochondrial D-loop mutations and deletion profiles of cancerous and noncancerous liver tissue in hepatitis B virus-infected liver. Br J Cancer, 2005, 92: 1268–1272, 10.1038/sj.bjc.6602496, 1:CAS:528:DC%2BD2MXivFWltL0%3D, 15785740
Yu M, Shi Y, Zhang F, et al. Sequence variations of mitochondrial DNA D-loop region are highly frequent events in familial breast cancer. J Biomed Sci, 2008, 15: 535–543, 10.1007/s11373-007-9229-4, 1:CAS:528:DC%2BD1cXptVWisLg%3D, 18157618
Chen G F, Chan F L, Hong B F, et al. Mitochondrial DNA mutations in chemical carcinogen-induced rat bladder and human bladder cancer. Oncol Rep, 2004, 12: 463–472, 1:CAS:528:DC%2BD2cXms1Kitbc%3D, 15254717
Zhou Jin, Meng Ran, Li Limin, et al. Mitochondrial D-loop mutations in patients with acute myeloid leukemia. Chin J Endemiol, 2006, 25: 90–93, 1:CAS:528:DC%2BD2sXksVaitb8%3D
Lu D P. The Iatreusiology of Leukemia. Beijing: The Beijing Science and Technology Publishing Company, 1990. 28–29
Ni J H, Chen G Q, Shen Z X, et al. Pharmacokinetics of intravenous arsenic trioxide in the treatment of acute promyelocytic leukemia. Chin J Hematol, 1997, 18: 250–253, 1:CAS:528:DyaK1cXmslegsw%3D%3D
Zhou J, Meng R, Yang B F. Comparing two arsenic trioxide administration methods in APL therapy. CMJ, 2004, 117: 1101–1104
Ingman M, Kaessmann H, Paabo S, et al. Mitochondrial genome variation and the origin of modern humans. Nature, 2000, 408: 708–713, 10.1038/35047064, 1:CAS:528:DC%2BD3cXptVCrsr4%3D, 11130070
Park I C, Park M J, Woo S H, et al. Tetraarsenic oxide induces apoptosis in U937 leukemic cells through a reactive oxygen species-dependent pathway. Int J Oncol, 2003, 23: 943–948, 1:CAS:528:DC%2BD3sXovV2gtbg%3D, 12963972
Zhou J, Meng R, Sui X H, et al. Effects of protein tyrosine kinase, protein tyrosine phosphatase and protein kinase C on the apoptosis of arsenic trioxide treated NB4 cells and human cortex neurons. Chin J Hematol, 2004, 25: 600–604
Zhou J, Meng R, Sui X H, et al. The effects of administration styles of arsenic trioxide on intracellular arsenic concentration, cell differentiation and apoptosis. Haematologica, 2005, 90: 1277–1279, 1:CAS:528:DC%2BD2MXhtFGrtLvO, 16154855
Zhou J, Meng R, Sui X H, et al. Various tolerances to arsenic trioxide between human cortical neurons and leukemic cells. Sci China Ser C-Life Sci, 2006, 49: 567–572, 10.1007/s11427-006-2034-x, 1:CAS:528:DC%2BD2sXitlOktw%3D%3D
Ishikawa K, Takenaga K, Akimoto M, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science, 2008, 320: 661–664, 10.1126/science.1156906, 1:CAS:528:DC%2BD1cXltFyisrc%3D, 18388260
Hung W Y, Lin J C, Lee L M, et al. Tandem duplication/triplication correlated with poly-cytosine stretch variation in human mitochondrial DNA D-loop regi93n. Mutagenesis, 2008, 23: 137–142, 10.1093/mutage/gen002, 1:CAS:528:DC%2BD1cXktlWht78%3D, 18252697
Kwong J Q, Henning M S, Starkov A A, et al.The mitochondrial respiratory chain is a modulator of apoptosis. J Cell Biol, 2007, 179: 1163–1177, 10.1083/jcb.200704059, 1:CAS:528:DC%2BD2sXhsVOgtL7P, 18086914
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Meng, R., Zhou, J., Sui, M. et al. Arsenic trioxide promotes mitochondrial DNA mutation and cell apoptosis in primary APL cells and NB4 cell line. Sci. China Life Sci. 53, 87–93 (2010). https://doi.org/10.1007/s11427-010-0004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-010-0004-9