Abstract
Purpose
Physiologically-based pharmacokinetic (PBPK) modeling offers a unique modality to predict age-specific pharmacokinetics. The objective of this study was to assess the ability of PBPK model to predict plasma exposure of oxycodone, a widely used opioid for pain management, in adults and children.
Methods
A full PBPK model of oxycodone following intravenous and oral administration was developed using a ‘bottom-up’ and ‘top-down’ combined strategy. The model was then extrapolated to pediatrics through a reasonable scaling method. The adult and pediatric model was evaluated using data from 17 clinical PK studies by testing predicted/observed goodness of fit. The mean fold error for PK parameters was calculated. Finally, we used the validated PBPK model to visualize adult-children dose conversion for oxycodone.
Results
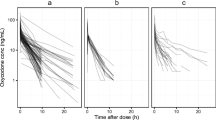
The developed PBPK model successfully predicted the oxycodone disposition in adults, wherein the predicted versus observed AUC, Cmax, and tmax were within 0.90 to 1.20-fold difference. After scaling anatomy/physiology, protein binding, and clearance, the model showed satisfactory prediction performance for pediatric populations as predicted AUC were within the 1.50-fold range of the observed values. According to the application of PBPK model, we found that different intravenous doses should be given in children of different ages compared to a standard 0.1 mg/kg in adults, while a progressive increasing dose with age growth following oral administration is recommended for children.
Conclusions
The current example provides the opportunity for using the PBPK model to guide dose adjustment of oxycodone in the design of future pediatric clinical studies.





Similar content being viewed by others
References
Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452(1–2):3–7.
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NHG. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Brit J Anaesth. 2004;92(2):208–17.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3:e150.
Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacok. 2009;24(1):25–36.
Luzon E, Blake K, Cole S, Nordmark A, Versantvoort C, Berglund EG. Physiologically based pharmacokinetic modeling in regulatory decision-making at the European medicines agency. Clin Pharmacol Ther. 2017;102(1):98–105.
Grimstein M, Yang Y, Zhang X, Grillo J, Huang SM, Zineh I, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration's Office of Clinical Pharmacology. J Pharm Sci. 2019;108(1):21–5.
Bianchi DW, Gillman MW. Addressing the impact of opioids on women and children. Am J Obstet Gynecol. 2019;221(2):123−+.
Biancofiore G. Oxycodone controlled release in cancer pain management. Ther Clin Risk Manag. 2006;2(3):229–34.
Kinnunen M, Piirainen P, Kokki H, Lammi P, Kokki M. Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet. 2019;58(6):705–25.
Lalovic B, Phillips B, Risler LL, Howald W, Shen DD. Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos. 2004;32(4):447–54.
Romand S, Spaggiari D, Marsousi N, Samer C, Desmeules J, Daali Y, et al. Characterization of oxycodone in vitro metabolism by human cytochromes P450 and UDP-glucuronosyltransferases. J Pharm Biomed Anal. 2017;144:129–37.
Davis MP, Varga J, Dickerson D, Walsh D, LeGrand SB, Lagman R. Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support Care Cancer. 2003;11(2):84–92.
Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79(5):461–79.
Marsousi N, Daali Y, Rudaz S, Almond L, Humphries H, Desmeules J, et al. Prediction of metabolic interactions with oxycodone via CYP2D6 and CYP3A inhibition using a physiologically based pharmacokinetic model. CPT Pharmacometrics Syst Pharmacol. 2014;3:e152.
Willmann S, Lippert J, Sevestre M, Solodenko J, Fois F, Schmitt W. PK-Sim (R): a physiologically based pharmacokinetic'whole-body'model. Biosilico. 2003;4(1):121–4.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Basit A, Radi Z, Vaidya VS, Karasu M, Prasad B. Kidney cortical transporter expression across species using quantitative proteomics. Drug Metab Dispos. 2019:dmd. 119.086579.
T'Jollyn H, Vermeulen A, Van Bocxlaer J. PBPK and its virtual populations: the impact of physiology on pediatric pharmacokinetic predictions of tramadol. AAPS J. 2018;21(1):8.
Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003;123(5):369–75.
Nieminen TH, Hagelberg NM, Saari TI, Pertovaara A, Neuvonen M, Laine K, et al. Rifampin greatly reduces the plasma concentrations of intravenous and oral oxycodone. Anesthesiology. 2009;110(6):1371–8.
Saari TI, Gronlund J, Hagelberg NM, Neuvonen M, Laine K, Neuvonen PJ, et al. Effects of itraconazole on the pharmacokinetics and pharmacodynamics of intravenously and orally administered oxycodone. Eur J Clin Pharmacol. 2010;66(4):387–97.
Gronlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Laine K, Olkkola KT. Effect of inhibition of cytochrome P450 enzymes 2D6 and 3A4 on the pharmacokinetics of intravenous oxycodone: a randomized, three-phase, crossover, placebo-controlled study. Clin Drug Investig. 2011;31(3):143–53.
Oshlack B, Chasin M, Minogue JJ, Kaiko RF. Controlled release oxycodone compositions. In: Google Patents; 1996.
Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol. 1972;24(12):979–81.
Li Y, Sun D, Palmisano M, Zhou S. Slow drug delivery decreased total body clearance and altered bioavailability of immediate- and controlled-release oxycodone formulations. Pharmacol Res Perspect. 2016;4(1):e00210.
Biesdorf C, Martins FS, Sy SKB, Diniz A. Physiologically-based pharmacokinetics of ziprasidone in pregnant women. Br J Clin Pharmacol. 2019;85(5):914–23.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4(1):E4.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45(7):683–704.
Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci. 2000;2(1):E3.
Kokki H, Rasanen I, Reinikainen M, Suhonen P, Vanamo K, Ojanpera I. Pharmacokinetics of oxycodone after intravenous, buccal, intramuscular and gastric administration in children. Clin Pharmacokinet. 2004;43(9):613–22.
El-Tahtawy A, Kokki H, Reidenberg BE. Population pharmacokinetics of oxycodone in children 6 months to 7 years old. J Clin Pharmacol. 2006;46(4):433–42.
Olkkola KT, Hamunen K, Seppala T, Maunuksela EL. Pharmacokinetics and ventilatory effects of intravenous oxycodone in postoperative children. Br J Clin Pharmacol. 1994;38(1):71–6.
Chen N, Aleksa K, Woodland C, Rieder M, Koren G. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21(2):160–8.
Mooij MG, de Koning BA, Huijsman ML, de Wildt SN. Ontogeny of oral drug absorption processes in children. Expert Opin Drug Metab Toxicol. 2012;8(10):1293–303.
Kuepfer L, Niederalt C, Wendl T, Schlender JF, Willmann S, Lippert J, et al. Applied concepts in PBPK modeling: how to build a PBPK/PD model. CPT Pharmacometrics Syst Pharmacol. 2016;5(10):516–31.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
Gronlund J, Saari T, Hagelberg N, Martikainen IK, Neuvonen PJ, Olkkola KT, et al. Effect of telithromycin on the pharmacokinetics and pharmacodynamics of oral oxycodone. J Clin Pharmacol. 2010;50(1):101–8.
Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, et al. The effects of CYP2D6 and CYP3A activities on the pharmacokinetics of immediate release oxycodone. Br J Pharmacol. 2010;160(4):907–18.
Fenneteau F, Poulin P, Nekka F. Physiologically based predictions of the impact of inhibition of intestinal and hepatic metabolism on human pharmacokinetics of CYP3A substrates. J Pharm Sci. 2010;99(1):486–514.
Marsousi N, Desmeules JA, Rudaz S, Daali Y. Prediction of drug-drug interactions using physiologically-based pharmacokinetic models of CYP450 modulators included in Simcyp software. Biopharm Drug Dispos. 2018;39(1):3–17.
Moore C, Kelley-Baker T, Lacey J. Interpretation of oxycodone concentrations in oral fluid. J Opioid Manag. 2012;8(3):161–6.
Poyhia R, Olkkola KT, Seppala T, Kalso E. The pharmacokinetics of oxycodone after intravenous injection in adults. Br J Clin Pharmacol. 1991;32(4):516–8.
Gronlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Olkkola KT, Laine K. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Brit J Clin Pharmaco. 2010;70(1):78–87.
Gronlund J, Saari TI, Hagelberg N, Neuvonen PJ, Olkkola KT, Laine K. Miconazole oral gel increases exposure to oral oxycodone by inhibition of CYP2D6 and CYP3A4. Antimicrob Agents Chemother. 2011;55(3):1063–7.
Hagelberg NM, Nieminen TH, Saari TI, Neuvonen M, Neuvonen PJ, Laine K, et al. Voriconazole drastically increases exposure to oral oxycodone. Eur J Clin Pharmacol. 2009;65(3):263–71.
Liukas A, Kuusniemi K, Aantaa R, Virolainen P, Neuvonen M, Neuvonen PJ, et al. Plasma concentrations of oral oxycodone are greatly increased in the elderly. Clin Pharmacol Ther. 2008;84(4):462–7.
Nieminen TH, Hagelberg NM, Saari TI, Neuvonen M, Neuvonen PJ, Laine K, et al. Grapefruit juice enhances the exposure to oral oxycodone. Basic Clin Pharmacol Toxicol. 2010;107(4):782–8.
Nieminen TH, Hagelberg NM, Saari TI, Neuvonen M, Laine K, Neuvonen PJ, et al. St John's wort greatly reduces the concentrations of oral oxycodone. Eur J Pain. 2010;14(8):854–9.
Mandema JW, Kaiko RF, Oshlack B, Reder RF, Stanski DR. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42(6):747–56.
Webster LR, Bath B, Medve RA, Marmon T, Stoddard GJ. Randomized, double-blind, placebo-controlled study of the abuse potential of different formulations of oral oxycodone. Pain Med. 2012;13(6):790–801.
Setnik B, Bass A, Bramson C, Levy-Cooperman N, Malhotra B, Matschke K, et al. Abuse potential study of ALO-02 (extended-release oxycodone surrounding sequestered naltrexone) compared with immediate-release oxycodone administered orally to nondependent recreational opioid users. Pain Med. 2017;18(6):1077–88.
Benziger DP, Miotto J, Grandy RP, Thomas GB, Swanton RE, Fitzmartin RD. A pharmacokinetic/pharmacodynamic study of controlled-release oxycodone. J Pain Symptom Manag. 1997;13(2):75–82.
Reder RF, Oshlack B, Miotto JB, Benziger DD, Kaiko RF. Steady-state bioavailability of controlled-release oxycodone in normal subjects. Clin Ther. 1996;18(1):95–105.
Acknowledgments and Disclosures
The authors thank current all members in Open Systems Pharmacology community in GitHub for providing proper instructions and advice during learning PBPK modeling. The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, L., Xu, M., Tang, Sw. et al. Physiologically Based Pharmacokinetic Modeling of Oxycodone in Children to Support Pediatric Dosing Optimization. Pharm Res 36, 171 (2019). https://doi.org/10.1007/s11095-019-2708-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2708-2