Abstract
Purpose
To investigate changes in the phosphorylation of myosin light chain (MLC) in response to histamine and its effect on the barrier integrity of corneal epithelial cells.
Materials and Methods
Experiments were performed in bovine corneal epithelial cells (BCEC). RT-PCR and Western blotting were employed to characterize expression of H1 receptors and MLC kinase (MLCK). Phosphorylation of MLC was assessed by urea-glycerol gel electrophoresis and Western blotting. Barrier integrity was determined as permeability to horseradish peroxidase (HRP; 44 kDa) across monolayers grown on porous filters.
Results
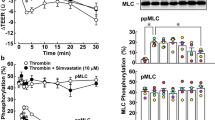
Expression of both H1 receptors and MLCK was found in BCEC. Exposure to histamine induced significant MLC phosphorylation concomitant with an increase in HRP permeability. In addition, organization of the cortical actin found in resting cells was disrupted. In contrast to histamine, ATP (a P2Y receptor agonist) induced dephosphorylation of MLC. Pre-exposure to ATP reduced the effect of histamine on HRP permeability and disruption of cortical actin.
Conclusion
MLC phosphorylation, a biochemical pre-requisite for increased contractility of the actin cytoskeleton, led to histamine-induced breakdown of the barrier integrity in the corneal epithelial cells. This is attributed to weakening of the tethering forces at the tight junctions by the centripetal forces produced by increased actin contractility.










Similar content being viewed by others
References
B. J. McLaughlin, R. B. Caldwell, Y. Sasaki, and T. O. Wood. Freeze-fracture quantitative comparison of rabbit corneal epithelial and endothelial membranes. Curr. Eye Res. 4:951–961 (1985).
Y. Wang, M. Chen, and J. M. Wolosin. ZO-1 in corneal epithelium; stratal distribution and synthesis induction by outer cell removal. Exp. Eye Res. 57:283–292 (1993).
J. M. Diamond. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist. 20:10–18 (1977).
B. Gumbiner. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. 253:C749–C758 (1987).
M. A. Behzadian, X. L. Wang, L. J. Windsor, N. Ghaly, and R. B. Caldwell. TGF-beta increases retinal endothelial cell perme?>ability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Investig. Ophthalmol. Vis. Sci. 42:853–859 (2001).
F. Hollande, E. M. Blanc, J. P. Bali, R. H. Whitehead, A. Pelegrin, G. S. Baldwin, and A. Choquet. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am. J. Physiol.: Gasterointest. Liver Physiol. 280:G910–G921 (2001).
S. V. Walsh, A. M. Hopkins, and A. Nusrat. Modulation of tight junction structure and function by cytokines. Adv. Drug Deliv. Rev. 41:303–313 (2000).
B. P. McNamara, A. Koutsouris, C. B. O’Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Invest. 107:621–629 (2001).
X. Yi, Y. Wang, and F. S. Yu. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Investig. Ophthalmol. Vis. Sci. 41:4093–4100 (2000).
M. Itoh, M. Furuse, K. Morita, K. Kubota, M. Saitou, and S. Tsukita. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 147:1351–1363 (1999).
A. Nusrat, J. R. Turner, and J. L. Madara. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am. J. Physiol.: Gasterointest. Liver Physiol. 279:G851–G857 (2000).
J. G. Garcia, H. W. Davis, and C. E. Patterson. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J. Cell. Physiol. 163:510–522 (1995).
J. R. Turner. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin. Cell Dev. Biol. 11:301–308 (2000).
S. M. Dudekand and J. G. Garcia. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 91:1487–1500 (2001).
H. Lumand and A. B. Malik. Regulation of vascular endothelial barrier function. Am. J. Physiol. 267:L223–L241 (1994).
M. Satpathy, P. Gallagher, M. Lizotte-Waniewski, and S. P. Srinivas. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp. Eye Res. 79:477–486 (2004).
R. Yuhan, A. Koutsouris, S. D. Savkovic, and G. Hecht. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 113:1873–1882 (1997).
Y. Zolotarevsky, G. Hecht, A. Koutsouris, D. E. Gonzalez, C. Quan, J. Tom, R. J. Mrsny, and J. R. Turner. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease.[erratum appears in Gastroenterology 2002 Oct;123(4):1412]. Gastroenterology 123:163–172 (2002).
L. Shen, E. D. Black, E. D. Witkowski, W. I. Lencer, V. Guerriero, E. E. Schneeberger, and J. R. Turner. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 119:2095–2106 (2006).
K. E. Kammand and J. T. Stull. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 276:4527–4530 (2001).
A. P. Somlyo and A. V. Somlyo. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83:1325–1358 (2003).
A. B. Moy, J. Van Engelenhoven, J. Bodmer, J. Kamath, C. Keese, I. Giaever, S. Shasby, and D. M. Shasby. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J. Clin. Invest. 97:1020–1027 (1996).
G. P. van Nieuw Amerongen, R. Draijer, M. A. Vermeer, and V. W. van Hinsbergh. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ. Res. 83:1115–1123 (1998).
S. P. Srinivas, M. Satpathy, Y. Guo, and V. Anandan. Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 47:4011–4018 (2006).
A. Leonardi. The central role of conjunctival mast cells in the pathogenesis of ocular allergy. Curr. Allergy Asthma Rep. 2:325–331 (2002).
L. Bieloryand and S. Ghafoor. Histamine receptors and the conjunctiva. Curr. Opin. Allergy Clin. Immunol. 5:437–440 (2005).
T. Noll, M. Schafer, U. Schavier-Schmitz, and H. M. Piper. ATP induces dephosphorylation of myosin light chain in endothelial cells. Am. J. Physiol., Cell Physiol. 279:C717–C723 (2000).
M. Satpathy, P. Gallagher, Y. Jin, and S. P. Srinivas. Extracellular ATP opposes thrombin-induced myosin light chain phosphorylation and loss of barrier integrity in corneal endothelial cells. Exp. Eye Res. 81:183–192 (2005).
S. P. Srinivas, M. Satpathy, P. Gallagher, E. Lariviere, and W. Van Driessche. Adenosine induces dephosphorylation of myosin II regulatory light chain in cultured bovine corneal endothelial cells. Exp. Eye Res. 79:543–551 (2004).
A. P. Somlyo and A. V. Somlyo. Signal transduction and regulation in smooth muscle.[erratum appears in Nature 1994 Dec 22–29;372(6508):812]. Nature 372:231–236 (1994).
V. Lazarand and J. G. Garcia. A single human myosin light chain kinase gene (MLCK; MYLK). Genomics 57:256–267 (1999).
K. Kimura, M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, J. Feng, T. Nakano, K. Okawa, A. Iwamatsu, and K. Kaibuchi. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase)[see comment]. Science 273:245–248 (1996).
K. M. Crawford, D. K. MacCallum, and S. A. Ernst. Histamine H1 receptor-mediated Ca2+ signaling in cultured bovine corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 33:3041–3049 (1992).
K. M. Crawford, D. K. MacCallum, and S. A. Ernst. Agonist-induced Ca2+ mobilization in cultured bovine and human corneal endothelial cells. Curr. Eye Res. 12:303–311 (1993).
N. A. Sharif, T. K. Wiernas, W. E. Howe, B. W. Griffin, E. A. Offord, and A. M. Pfeifer. Human corneal epithelial cell functional responses to inflammatory agents and their antagonists. Investig. Ophthalmol. Vis. Sci. 39:2562–2571 (1998).
A. Kittel, E. Kaczmarek, J. Sevigny, K. Lengyel, E. Csizmadia, and S. C. Robson. CD39 as a caveolar-associated ectonucleotidase. Biochem. Biophys. Res. Commun. 262:596–599 (1999).
A. J. Marcus, M. J. Broekman, J. H. Drosopoulos, N. Islam, D. J. Pinsky, C. Sesti, and R. Levi. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J. Pharmacol. Exp. Ther. 305:9–16 (2003).
T. Hashikawa, M. Takedachi, M. Terakura, T. Saho, S. Yamada, L. F. Thompson, Y. Shimabukuro, and S. Murakami. Involvement of CD73 (ecto-5′-nucleotidase) in adenosine generation by human gingival fibroblasts. J. Dent. Res. 82:888–892 (2003).
M. Eto, T. Ohmori, M. Suzuki, K. Furuya, and F. Morita. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J. Biochem. 118:1104–1107 (1995).
M. Eto, S. Senba, F. Morita, and M. Yazawa. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 410:356–360 (1997).
S. M. Bloemers, S. Verheule, M. P. Peppelenbosch, M. J. Smit, L. G. Tertoolen, and S. de Laat. Sensitization of the histamine H1 receptor by increased ligand affinity. J. Biol. Chem. 273:2249–2255 (1998).
T. Noll, H. Holschermann, K. Koprek, D. Gunduz, W. Haberbosch, H. Tillmanns, and H. M. Piper. ATP reduces macromolecule permeability of endothelial monolayers despite increasing [Ca2+]i. Am. J. Physiol. 276:H1892–H1901 (1999).
J. Qiao, F. Huang, and H. Lum. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am. J. Physiol., Lung Cell. Mol. Physiol. 284:L972–L980 (2003).
G. Burnstock. P2 purinoceptors: historical perspective and classification. Ciba Found. Symp. 198:1–28; discussion 29–34 (1996).
K. Enomoto, K. Furuya, S. Yamagishi, T. Oka, and T. Maeno. The increase in the intracellular Ca2+ concentration induced by mechanical stimulation is propagated via release of pyrophosphorylated nucleotides in mammary epithelial cells. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 427:533–542 (1994).
J. R. Turner, B. K. Rill, S. L. Carlson, D. Carnes, R. Kerner, R. J. Mrsny, and J. L. Madara. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. 273:C1378–C1385 (1997).
Acknowledgements
Supported by VISTAKON Research Grant, American Optometric Foundation, 2004 (SPS) and NEI 14415 (SPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Y., Ramachandran, C., Satpathy, M. et al. Histamine-induced Myosin Light Chain Phosphorylation Breaks Down the Barrier Integrity of Cultured Corneal Epithelial Cells. Pharm Res 24, 1824–1833 (2007). https://doi.org/10.1007/s11095-007-9309-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9309-1