Abstract
Purpose
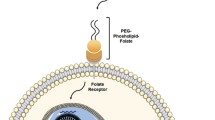
Development of a polyethylene glycol (PEG)-stabilized immunoliposome (PSIL) formulation with high DNA content suitable for in vivo intravenous administration and targeted gene delivery.
Materials and Methods
Plasmid DNA was condensed using 40% ethanol and packaged into neutral PSILs targeted to the mouse transferrin receptor using monoclonal antibodies (MAbs; clones RI7 and 8D3) attached to their PEG maleimide moieties. PSILs size was measured by quasi-elastic light scattering. The targeting capacity of the formulation was determined by transfection of mouse neuroblastoma Neuro 2A (N2A) cells with PSIL-DNA complexes conjugated with either RI7 or 8D3 MAbs.
Results
DNA encapsulation and MAb conjugation efficiencies averaged 71 ± 14% and 69 ± 5% (mean ± SD), respectively. No alteration in mean particle size (< 100 nm) or DNA leakage were found after 48 h storage in a physiological buffer, and the in vivo terminal half-life reached 23.9 h, indicating that the PSIL-DNA formulation was stable. Addition of free RI7 MAbs prevented transfection of N2A cells with PSIL-DNA complexes conjugated with either RI7 or 8D3 MAbs, confirming that the transfection was transferrin receptor-dependent.
Conclusions
The present data suggest that our new PSIL formulation combines molecular features required for targeted gene therapy including high DNA encapsulation efficiencies and vector-specific transient transfection capacity.






Similar content being viewed by others
Abbreviations
- 2I:
-
2-iminothiolane
- AAV:
-
adeno-associated virus
- ATP:
-
adenosine 5′-triphosphate
- AV:
-
adenovirus
- BSA:
-
bovine serum albumine
- CMV:
-
cytomegalovirus
- [33P]-dCTP:
-
deoxycytidine 5′-[α-33P]triphosphate
- DDAB:
-
didodecyldimethylammonium bromide
- Dpm:
-
disintegrations per minute
- DSPE:
-
distearoylphosphatidylethanolamine
- EtOH:
-
ethanol
- HEPES:
-
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid
- HSV-1:
-
herpes simplex virus-1
- LSC:
-
liquid scintillation counting
- MAbs:
-
monoclonal antibodies
- MWCO:
-
molecular weight cut-off
- N2A:
-
neuroblastoma neuro 2A
- NC:
-
non-conjugated
- 3H-NSP:
-
N-succinimidyl-[2,3-3H] propionate
- PBS:
-
phosphate buffered saline
- PCR:
-
polymerase chain reaction
- pGLuc:
-
pCMV-GLuc
- PEG:
-
polyethylene glycol
- PEG2000 :
-
2,000 da polyethylene glycol
- POPC:
-
1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine
- PSLs:
-
PEG-stabilized liposomes
- PSILs:
-
PEG-stabilized immunoliposomes
- RLU:
-
relative light units
- RT:
-
room temperature
- QELS:
-
quasi-elastic light scattering
- SEM:
-
standard error mean
- SCID:
-
severe combined immunodeficiency
- SD:
-
standard deviation
- SV40:
-
simian virus 40
- VP-SFM:
-
virus production serum-free medium
References
M. G. Kaplitt and D. W. Pfaff. Viral vectors for gene delivery and expression in the CNS. Methods 10:343–350 (1996).
J. H. Kordower, M. E. Emborg, J. Bloch, S. Y. Ma, Y. Chu, L. Leventhal, J. McBride, E. Y. Chen, S. Palfi, B. Z. Roitberg, W. D. Brown, J. E. Holden, R. Pyzalski, M. D. Taylor, P. Carvey, Z. Ling, D. Trono, P. Hantraye, N. Deglon, and P. Aebischer. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290:767–773 (2000).
B. L. Davidson and X. O. Breakefield. Viral vectors for gene delivery to the nervous system. Nat. Rev., Neurosci. 4:353–364 (2003).
P. R. Lowenstein and M. G. Castro. Recent advances in the pharmacology of neurological gene therapy. Curr. Opin. Pharmacol. 4:91–97 (2004).
P. L. Sinn, S. L. Sauter, and P. B. McCray, Jr. Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors—design, biosafety, and production. Gene Ther. 12:1089–1098 (2005).
C. E. Thomas, A. Ehrhardt, and M. A. Kay. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev., Genet. 4:346–358 (2003).
K. Jooss and N. Chirmule. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 10:955–963 (2003).
M. A. Schnell, Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Molec. Ther. 3:708–722 (2001).
S. Hacein-Bey-Abina, C. Von Kalle, M. Schmidt, M. P. McCormack, N. Wulffraat, P. Leboulch, A. Lim, C. S. Osborne, R. Pawliuk, E. Morillon, R. Sorensen, A. Forster, P. Fraser, J. I. Cohen, G. de Saint Basile, I. Alexander, U. Wintergerst, T. Frebourg, A. Aurias, D. Stoppa-Lyonnet, S. Romana, I. Radford-Weiss, F. Gross, F. Valensi, E. Delabesse, E. Macintyre, F. Sigaux, J. Soulier, L. E. Leiva, M. Wissler, C. Prinz, T. H. Rabbitts, F. Le Deist, A. Fischer, and M. Cavazzana-Calvo. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302:415–419 (2003).
D. J. Glover, H. J. Lipps, and D. A. Jans. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev., Genet. 6:299–310 (2005).
N. Smyth Templeton. Liposomal delivery of nucleic acids in vivo. DNA Cell Biol. 21:857–867 (2002).
T. M. Allen and P. R. Cullis. Drug delivery systems: entering the mainstream. Science 303:1818–1822 (2004).
D. J. Bharali, I. Klejbor, E. K. Stachowiak, P. Dutta, I. Roy, N. Kaur, E. J. Bergey, P. N. Prasad, and M. K. Stachowiak. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. U. S. A. 102:11539–11544 (2005).
P. H. Tan, M. Manunta, N. Ardjomand, S. A. Xue, D. F. Larkin, D. O. Haskard, K. M. Taylor, and A. J. George. Antibody targeted gene transfer to endothelium. J. Gene Med. 5:311–323 (2003).
Y. Zhang, H. Jeong Lee, R. J. Boado, and W. M. Pardridge. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J. Gene Med. 4:183–194 (2002).
P. Machy, F. Lewis, L. McMillan, and Z. L. Jonak. Gene transfer from targeted liposomes to specific lymphoid cells by electroporation. Proc. Natl. Acad. Sci. U. S. A. 85:8027–8031 (1988).
Y. Zhang, F. Calon, C. Zhu, R. J. Boado, and W. M. Pardridge. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum. Gene Ther. 14:1–12 (2003).
N. Shi, Y. Zhang, C. Zhu, R. J. Boado, and W. M. Pardridge. Brain-specific expression of an exogenous gene after i.v. administration. Proc. Natl. Acad. Sci. U. S. A. 98:12754–12759 (2001).
C. Y. Wang and L. Huang. pH-sensitive immunoliposomes mediate target-cell-specific delivery and controlled expression of a foreign gene in mouse. Proc. Natl. Acad. Sci. U. S. A. 84:7851–7855 (1987).
N. Zhu, D. Liggitt, Y. Liu, and R. Debs. Systemic gene expression after intravenous DNA delivery into adult mice. Science 261:209–211 (1993).
A. Gabizon, H. Shmeeda, and Y. Barenholz. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 42:419–436 (2003).
P. R. Cullis, A. Chonn, and S. C. Semple. Interactions of liposomes and lipid-based carrier systems with blood proteins: relation to clearance behaviour in vivo. Adv. Drug Deliv. Rev. 32:3–17 (1998).
K. Maruyama, O. Ishida, T. Takizawa, and K. Moribe. Possibility of active targeting to tumor tissues with liposomes. Adv. Drug Deliv. Rev. 40:89–102 (1999).
A. L. Klibanov, K. Maruyama, V. P. Torchilin, and L. Huang. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268:235–237 (1990).
A. Schnyder and J. Huwyler. Drug transport to brain with targeted liposomes. NeuroRx 2:99–107 (2005).
P. Goyal, K. Goyal, S. G. Kumar, A. Singh, O. P. Katare, and D. N. Mishra. Liposomal drug delivery systems—clinical applications. Acta Pharm. 55:1–25 (2005).
N. Shi and W. M. Pardridge. Noninvasive gene targeting to the brain. Proc. Natl. Acad. Sci. U. S. A. 97:7567–7572 (2000).
L. Cattel, M. Ceruti, and F. Dosio. From conventional to stealth liposomes: a new frontier in cancer chemotherapy. Tumori 89:237–249 (2003).
R. I. Mahato, K. Kawabata, Y. Takakura, and M. Hashida. in vivo disposition characteristics of plasmid DNA complexed with cationic liposomes. J. Drug Target. 3:149–157 (1995).
G. Osaka, K. Carey, A. Cuthbertson, P. Godowski, T. Patapoff, A. Ryan, T. Gadek, and J. Mordenti. Pharmacokinetics, tissue distribution, and expression efficiency of plasmid [33P]DNA following intravenous administration of DNA/cationic lipid complexes in mice: use of a novel radionuclide approach. J. Pharm. Sci. 85:612–618 (1996).
L. D. Leserman, P. Machy, and J. Barbet. Cell-specific drug transfer from liposomes bearing monoclonal antibodies. Nature 293:226–228 (1981).
P. A. Monnard, T. Oberholzer, and P. Luisi. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta 1329:39–50 (1997).
N. H. Kim, H. M. Park, S. Y. Chung, E. J. Go, and H. J. Lee. Immunoliposomes carrying plasmid DNA: preparation and characterization. Arch. Pharm. Res. 27:1263–1269 (2004).
D. D. Lasic, B. Ceh, M. C. Stuart, L. Guo, P. M. Frederik, and Y. Barenholz. Transmembrane gradient driven phase transitions within vesicles: lessons for drug delivery. Biochim. Biophys. Acta 1239:145–156 (1995).
M. L. Lee, W. Y. Poon, and H. S. Kingdon. A two-phase linear regression model for biologic half-life data. J. Lab. Clin. Med. 115:745–748 (1990).
I. J. Hildebrandt, M. Iyer, E. Wagner, and S. S. Gambhir. Optical imaging of transferrin targeted PEI/DNA complexes in living subjects. Gene Ther. 10:758–764 (2003).
R. R. Nixon. Prion-associated increases in Src-family kinases. J. Biol. Chem. 280:2455–2462 (2005).
A. L. Bailey and S. M. Sullivan. Efficient encapsulation of DNA plasmids in small neutral liposomes induced by ethanol and calcium. Biochim. Biophys. Acta 1468:239–252 (2000).
Y. Fang, T. S. Spisz, and J. H. Hoh. Ethanol-induced structural transitions of DNA on mica. Nucleic Acids Res. 27:1943–1949 (1999).
A. Cudd and C. Nicolau. Intracellular fate of liposome-encapsulated DNA in mouse liver. Analysis using electron microscope autoradiography and subcellular fractionation. Biochim. Biophys. Acta 845:477–491 (1985).
L. B. Jeffs, L. R. Palmer, E. G. Ambegia, C. Giesbrecht, S. Ewanick, and I. MacLachlan. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm. Res. 22:362–372 (2005).
J. Huwyler, D. Wu, and W. M. Pardridge. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. U. S. A. 93:14164–14169 (1996).
Z. M. Qian, H. Li, H. Sun, and K. Ho. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 54:561–587 (2002).
W. A. Jefferies, M. R. Brandon, S. V. Hunt, A. F. Williams, K. C. Gatter, and D. Y. Mason. Transferrin receptor on endothelium of brain capillaries. Nature 312:162–163 (1984).
D. C. Mash, J. Pablo, D. D. Flynn, S. M. Efange, and W. J. Weiner. Characterization and distribution of transferrin receptors in the rat brain. J. Neurochem. 55:1972–1979 (1990).
R. J. Boado and W. M. Pardridge. Ten nucleotide cis element in the 3′-untranslated region of the GLUT1 glucose transporter mRNA increases gene expression via mRNA stabilization. Mol. Brain Res. 59:109–113 (1998).
Acknowledgements
Grants from the Canadian Institutes of Health Research (CIHR) (FC-RMP72549 and M2C-63922), the Alzheimer Society Canada (FC-ASC 0516), and the Parkinson Society Canada (FC 2004) funded this research. VR was supported by FORMSAV-CIHR and Laval University “Fonds d’Enseignement et de Recherche” faculty of pharmacy studentships.
Competing interests statement
The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Rivest, Phivilay, contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rivest, V., Phivilay, A., Julien, C. et al. Novel Liposomal Formulation for Targeted Gene Delivery. Pharm Res 24, 981–990 (2007). https://doi.org/10.1007/s11095-006-9224-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9224-x