No Heading
Purpose.
To evaluate the in vivo efficacy and pharmacokinetics of vancomycin delivered from glycerylmonostearate (GMS) implants in a prosthetic-device based biofilm infection model.
Methods.
A biofilm infection model was developed in male Sprague-Dawley rats by implanting a vascular graft on the dorsal side of each rat and infecting it with 1.5 × 108 cfu/ml Staphylococcus epidermidis. The rats were divided into 3 groups of 6 rats each: 1) the control group that received no antibiotics, 2) the IM group that received multiple IM injections of vancomycin at a dose of 25 mg/kg every 6 h for a total of 12 doses, and 3) the implant group that received GMS implants designed to deliver vancomycin at a total dose of 300 mg/kg for a period of 4 days. The pharmacokinetics of vancomycin was determined from IM and implant groups by analyzing for vancomycin in blood using HPLC. In vivo efficacy was studied by evaluation of the wound site and the prosthetic device upon excision, for evidence of infection in the form of purulent discharge at the wound site and yellowish discoloration of the prosthetic device and inflammation as sign of biofilm formation. Microbiological evaluation on the wound site and the prosthetic device was performed by culturing the swabs at the wound site and the prosthetic device in sterile tryptic soy broth for 36–48 h at 37°C.
Results.
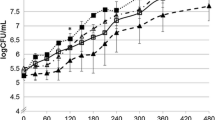
Vancomycin was successfully delivered in a sustained manner for 100 h from GMS implants and the resulting plasma profile showed that the concentrations, after an initial burst, plateaued at about of 4.77 ± 1.43 μg/ml with less fluctuations than the IM group in which the plasma concentrations fluctuated between 2.73 ± 0.94 μg/ml and 19.26 ± 3.67 μg/ml. Upon excision of the wound site, all the animals in the control group developed infection in the form of purulent discharge and yellowish discoloration of the prosthetic device. However, none of the rats in the implant group showed evidence of infection clearly demonstrating the efficacy of the local delivery system in preventing infection. Systemically delivered vancomycin by IM injections failed to prevent infection in four out of six rats. Microbiological evaluation of the wound site and prosthetic device resulted in isolation of biofilm-producing organisms such as Staphylococcus epidermidis, Enterococcus faecalis, and Staphylococcus aureus. These organisms were isolated in greater number of animals in the control group compared to the IM and implant groups.
Conclusions.
The GMS implants as a delivery system for vancomycin were successful in preventing infection in all the animals compared to the IM and control groups demonstrating the efficacy of a local delivery system in a prosthetic device related biofilm infection model.
Similar content being viewed by others
References
1. R. Langer and D. A. Tirrell. Designing materials for biology and medicine. Nature 428:487–492 (2004).
2. W. Zimmerli and F. A. Waldvogel. Models of foreign-body infections. In O. Zak and M. A. Sande, (eds.), Experimental Models in Antimicrobial Chemotherapy, 1986, 1, Academic Press, London, UK, pp. 295–317.
3. M. Dickinson and A. L. Bisno. Infections associated with indwelling implants: concepts of pathogenesis; infections associated with intravascular implants. Antimicrob. Agents Chemother. 33:597–601 (1989).
4. C. Potera. Biofilms invade microbiology. Science 273:1795–1797 (1996).
5. F. A. Waldvogel, P. E. Vaudaux, D. Pittet, and P. D. Lew. Perioperative antibiotic prophylaxis of wound and foreign body infections: microbial factors affecting efficacy. Rev. Infect. Dis. 13:S782–S789 (1991).
6. J. Costerton. Overview of microbial biofilms. J. Ind. Microbiol. 15:137–140 (1995).
7. T. Jouenne, O. Tresse, and J. Junter. Agar-entrapped bacteria as an in vitro model of biofilms and their susceptibility to antibiotics. FEMS Microbiol. Lett. 119:237–242 (1994).
8. M. R. W. Brown and P. Gilbert. Sensitivity of Biofilms to antimicrobial agents, J. Applied Bacteriol. Symp. Suppl. 74:87S–97S (1993).
9. J. C. Nickel, I. Ruseska, I. B. Wright, and J. B. Costerton. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619–624 (1985).
10. J. W. Costerton. The Etiology and persistence of cryptic bacterial infections: a hypothesis. Rev. Infect. Dis. 6:S606–S618 (1984).
11. M. R. Weitekamp and G. M. Caputo. Antibiotic prophylaxis: update on common clinical uses. Am. Fam. Physician 48:597–604 (1993).
12. K. Adams, L. Couch, G. Cierny, J. Calhoun, and J. T. Mader. In vitro and In vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin. Orthoped. Rel. Res. 278:244–252 (1992).
13. K. J. Wilson, G. Cierny, K. R. Adams, and J. T. Mader. Comparative evaluation of the diffusion of tobramycin and cefotaxime out of antibiotic-impregnated polymethylmethacrylate beads. J. Orthop. Res. 6:279–286 (1988).
14. K. Adams, L. Couch, G. Cierny, J. Calhoun, and J. T. Mader. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin. Orthop. Rel. Res. 278:244–252 (1992).
15. J. C. Calhoun and J. T. Mader. Antibiotic beads in the management of surgical infections. Am. J. Surg. 157:443–449 (1989).
16. C. T. Laurencin, T. Gerhart, P. Witschger, R. Satcher, A. Domb, A. E. Rosenberg, P. Hanff, L. Edsberg, W. Hayes, and R. Langer. Bioerodible polyanhydrides for antibiotic drug delivery: in vivo osteomyelitis treatment in a rat model system. J. Orthop. Res. 11:627–632 (1993).
17. G. Wei, Y. Kotoura, M. Oka, T. Yamamuro, R. Wada, S. Hyon, and Y. Ikada. A bioabsorbable delivery system for the treatment of osteomyelitis. J. Bone Joint Surgery 73-B:246–252 (1991).
18. S. Allababidi and J. C. Shah. Efficacy and pharmacokinetics of site-specific cefazolin delivery using biodegradable implants in the prevention of post-operative wound infections. Pharm. Res. 15:325–333 (1998).
19. K. A. Rodovald. Therapeutic considerations for infections caused by Staphylococcus epidermidis. Pharmacotherapy 8:14S–19S (1988).
20. D. M. Chilukuri and J. C. Shah. In vitro release kinetics and mechanism of vancomycin release from GMS implants for the prophylaxis of prosthetic device related infections. Pharm. Res. 15:S96 (1998).
21. J. Luska and A. Marusic. Rapid high-performance liquid chromatographic determination of vancomycin in human plasma. J. Chromat. B 667:277–281 (1995).
22. J. G. Wagner and E. Nelson. J. Pharm. Sci. 53:1392–1403 (1964).
23. T. D. Sanford and E. D. Colby. Effect of xylazine and ketamine on blood pressure, heart rate, and respiratory rate in rabbits. Lab. Anim. Sci. 30:519–523 (1980).
24. R. O. Darouiche, A. Dhir, A. J. Miller, G. C. Landon, I. I. Raad, and D. M. Musher. Vancomycin penetration into biofilm covering infected prosthesis and effect on bacteria. J. Infect. Dis. 170:720–723 (1994).
25. S. Schwank, Z. Rajacic, W. Zimmerli, and J. Blaser. Impact of bacterial biofilm formation on in vitro and in vivo activities of antibiotics. Antimicrob. Agents Chemother. 42:895–898 (1998).
26. G. D. Christensen, W. A. Simpson, A. L. Bisno, and E. H. Beachey. Experimental foreign body infections in mice challenged with slime producing Staphylococcus epidermidis. Infection and Immunity, 40(1):407–410 (1983).
27. T. M. Bergamini, T. M. McCurry, J. D. Bernard, K. L. Hoeg, R. A. Corpus, J. C. Peyton, K. R. Brittain, and W. G. Cheadle. Antibiotic efficacy against Staphylococcus epidermidis adherent to vascular grafts. J. Surg. Res. 60:3–6 (1996).
28. A. B. Kaiser. Antimicrobial prophylaxis in surgery. Medical Intelligence 315:1129–1138 (1986).
29. A. E. Khoury, K. Lam, B. Ellis, and J. W. Costerton. Prevention and control of bacterial infections associated with medical devices. ASAIO Journal 38(3):M174–M178 (1992).
30. G. Wang, S. Liu, S. W. Ueng, and E. Chan. The release of cefazolin and gentamicin from biodegradable PLA/PGA beads. Int. J. Pharmaceutics 273:203–212 (2004).
31. M. L. Zeckel and J. R. Woodworth. Vancomycin, A Clinical Overview in Glycopeptide Antibiotics, Ramakrishnan Nagarajan, (ed.), Glycopeptide Antibiotics, Marcel Dekker, New York, 1994, pp. 309–412.
32. M. McGuckin. MRSA and VRSA in wound care: accept the challenge. Adv. Skin Wound Care 17:93–95 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript represents the personal opinion of the author and does not necessarily represent the views or policies of the Food and Drug Administration.
Rights and permissions
About this article
Cite this article
Chilukuri, D., Shah, J. Local Delivery of Vancomycin for the Prophylaxis of Prosthetic Device-Related Infections. Pharm Res 22, 563–572 (2005). https://doi.org/10.1007/s11095-005-2497-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2497-7