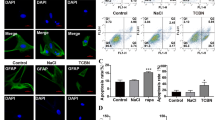
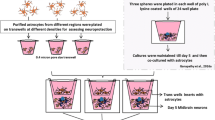
Abstract
Hypoxic stressors contribute to neuronal death in many brain diseases. Astrocyte processes surround most neurons and are therefore anatomically well-positioned to shield them from hypoxic injury. Excitatory amino acid transporters (EAATs), represent the sole mechanism of active reuptake of glutamate into the astrocytes and neurons and are essential to dampen neuronal excitation following glutamate release at synapses. Glutamate clearance impairment from any factors is bound to result in an increase in hypoxic neuronal injury. The brain energy metabolism under hypoxic conditions depends on monocarboxylate transporters (MCTs) that are expressed by neurons and glia. Previous co-immunoprecipitation experiments revealed that MCT4 directly modulate EAAT1 in astrocytes. The reduction in both surface proteins may act synergistically to induce neuronal hyperexcitability and excitotoxicity. Therefore we hypothesized that astrocytes would respond to hypoxic conditions by enhancing their expression of MCT4 and EAAT1, which, in turn, would enable them to better support neurons to survive lethal hypoxia injury. An oxygen deprivation (OD) protocol was used in primary cultures of neurons, astrocytes, and astrocytes–neurons derived from rat hippocampus, with or without MCT4-targeted short hairpin RNA (shRNA) transfection. Cell survival, expression of MCT4, EAAT1, glial fibrillary acidic protein and neuronal nuclear antigen were evaluated. OD resulted in significant cell death in neuronal cultures and up-regulation of MCT4, EAAT1 expression respectively in primary cell cultures, but no injury in neuron–astrocyte co-cultures and astrocyte cultures. However, neuronal cell death in co-cultures was increased exposure to shRNA-MCT4 prior to OD. These findings demonstrate that the MCT4-mediated expression of EAAT1 is involved in the resistance to hypoxia injury in astrocyte–neuron co-cultures.





Similar content being viewed by others
References
Pelvig DP, Pakkenberg H, Stark AK et al (2008) Neocortical glial cell numbers in human brains. Neurobiol Aging 29:1754–1762
Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Dienel GA, Cruz NF (2004) Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int 45:321–351
Kawahara K, Kosugi T, Tanaka M, Nakajima T, Yamada T (2005) Reversed operation of glutamate transporter GLT-1 is crucial to the development of preconditioning-induced ischemic tolerance of neurons in neuron/astrocyte co-cultures. Glia 49:349–359
Zhang M, Li WB, Geng JX, Li QJ, Sun XC, Xian XH, Qi J, Li SQ (2007) The upregulation of glial glutamate transporter-1 participates in the induction of brain ischemic tolerance in rats. J Cereb Blood Flow Metab 27:1352–1368
Weller ML, Stone IM, Goss A, Rau T, Rova C, Poulsen DJ (2008) Selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate but not severe hypoxia–ischemia. Neuroscience 155:1204–1211
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Halestrap AP, Meredith D (2004) The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflug Arch 447:619–628
Pellerin L, Bergersen LH, Halestrap AP, Pierre K (2005) Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res 79:55–64
Liu B, Niu L, Shen MZ, Gao L, Wang C, Li J, Song LJ, Tao Y, Meng Q, Yang QL, Gao GD, Zhang H (2014) Decreased astroglial monocarboxylate transporter 4 expression in temporal lobe epilepsy. Mol Neurobiol 50:327–338
Gao C, Wang C, Liu B, Wu H, Yang Q, Jin J, Li H, Dong S, Gao G, Zhang H (2014) Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes. Neurochem Res 39:2160–2169
Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC (1995) Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15:1835–1853
Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW (1994) Localization of neuronal and glial glutamate transporters. Neuron 13:713–725
Arriza JL, Eliasof S, Kavanaugh MP, Amara SG (1997) Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94:4155–4160
Jackman NA, Uliasz TF, Hewett JA, Hewett SJ (2010) Regulation of system x(c)(-)activity and expression in astrocytes by interleukin-1β: implications for hypoxic neuronal injury. Glia 58:1806–1815
Liu B, Hong JS (2003) Primary rat mesencephalic neuron-glia, neuron-enriched, microglia-enriched, and astroglia-enriched cultures. Methods Mol Med 79:387–395
McLendon RE, Bigner DD (1994) Immunohistochemistry of the glial fibrillary acidic protein: basic and applied considerations. Brain Pathol 4:221–228
Qin XF, An DS, Chen IS, Baltimore D (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA 100:183–188
Gao CJ, Niu L, Ren PC, Wang W, Zhu C, Li YQ, Chai W, Sun XD (2012) Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience 202:352–362
Nedergaard M, Dirnagl U (2005) Role of glial cells in cerebral ischemia. Glia 50:281–286
Rosenberg PA, Aizenman E (1989) Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett 103:162–168
Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916
Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L (2003) Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 37:275–286
Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513:532–541
Vibulsreth S, Hefti F, Ginsberg MD, Dietrich WD, Busto R (1987) Astrocytes protect cultured neurons from degeneration induced by anoxia. Brain Res 422:303–311
Cui W, Allen ND, Skynner M, Gusterson B, Clark AJ (2001) Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia 34:272–282
Dhandapani KM, Hadman M, De Sevilla L, Wade MF, Mahesh VB, Brann DW (2003) Astrocyte protection of neurons: role of transforming growth factor-beta signaling via a c-Jun-AP-1 protective pathway. J Biol Chem 278:43329–43339
Wang XF, Cynader MS (2001) Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Eurosci 21:3322–3331
Drukarch B, Schepens E, Stoof JC, Langeveld CH, Van Muiswinkel FL (1998) Astrocyte-enhanced neuronal survival is mediated by scavenging of extracellular reactive oxygen species. Free Radic Biol Med 25:217–220
Griffin S, Clark JB, Canevari L (2005) Astrocyte–neurone communication following oxygen glucose deprivation. J Neurochem 95:1015–1022
Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481
Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG (2010) Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 58:1042–1049
Kusumoto M, Dux E, Hossmann KA (1997) Effect of trophic factors on delayed neuronal death induced by in vitro ischemia in cultivated hippocampal and cortical neurons. Metab Brain Dis 12:113–120
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Vangeison G, Carr D, Federoff HJ, Rempe DA (2008) The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1 alpha in neurons and astrocytes. J Neurosci 28:1988–1993
Vilchez D, Ros S, Cifuentes D, Pujadas L, Vallès J, García-Fojeda B, CriadoGarcía O, Fernández-Sánchez E, Medraño-Fernández I, Domínguez J, García-Rocha M, Soriano E, Rodríguez de Córdoba S, Guinovart JJ (2007) Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10:1407–1413
Pellegri G, Rossier C, Magistretti PJ, Martin JL (1996) Cloning, localization and induction of mouse brain glycogen synthase. Brain Res Mol Brain Res 38:191–199
Hamprecht B, Verleysdonk S, Wiesinger H (2005) Enzymes of carbohydrate and energy metabolism. In: Kettenmann H, Ransom BR (eds) Neuroglia, 2nd edn. Oxford University Press, New York, pp 202–215
Zemke D, Smith JL, Reeves MJ, Majid A (2004) Ischemia and ischemic tolerance in the brain: an overview. Neurotoxicology 25:895–904
Dirnagl U, Meisel A (2008) Endogenous neuroprotection: mito-chondria as gateways to cerebral preconditioning? Neuropharmacology 55:334–344
Schaller B, Graf R (2002) Cerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention ? J Neurol 249:1503–1511
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254
Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T (2011) Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis 41:577–584
Lauritzen F, Heuser K, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Gjedde A, Eid T, Bergersen LH (2012) Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia 60:1172–1181
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Olney JW, Collins RC, Sloviter RS (1986) Excitotoxic mechanisms of epileptic brain damage. Adv Neurol 44:857–877
Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM (2005) Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57:226–235
Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702
Dienel GA, Cruz NF (2008) Imaging brain activation: simple pictures of complex biology. Ann NY Acad Sci 1147:139–170
Acknowledgments
The authors thank M.M. Qiang Zhang for help with the primary cell culture protocol and Yuhu Liu for his contributions to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Chen Gao and Wenxia Zhu Contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, C., Zhu, W., Tian, L. et al. MCT4-Mediated Expression of EAAT1 is Involved in the Resistance to Hypoxia Injury in Astrocyte–Neuron co-Cultures. Neurochem Res 40, 818–828 (2015). https://doi.org/10.1007/s11064-015-1532-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1532-2