Abstract
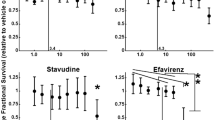
In active antiretroviral therapy antiretroviral drugs are employed for the restoration of a functional immune system in patients suffering from the acquired immunodeficiency syndrome. However, potential adverse effects of such compounds to brain cells are discussed in connection with the development of neurocognitive impairments in patients. To investigate potential effects of antiretroviral drugs on cell viability and the glycolytic flux of brain cells, astrocyte-rich primary cultures were exposed to various antiretroviral compounds, including the non-nucleoside reverse transcriptase inhibitor efavirenz. In a concentration of 10 μM, neither efavirenz nor any of the other investigated antiretroviral compounds acutely compromised the cell viability nor altered glucose consumption or lactate production. In contrast, the primary metabolite of efavirenz, 8-hydroxy-efavirenz, stimulated the glycolytic flux in viable astrocytes in a time- and concentration-dependent manner with half-maximal and maximal effects at concentrations of 5 and 10 μM, respectively. The stimulation of glycolytic flux by 8-hydroxy-efavirenz was not additive to that obtained for astrocytes that were treated with the respiratory chain inhibitor rotenone and was abolished by removal of extracellular 8-hydroxy-efavirenz. In a concentration of 10 μM, 8-hydroxy-efavirenz and efavirenz did not affect mitochondrial respiration, while both compounds lowered in a concentration of 60 μM significantly the oxygen consumption by mitochondria that had been isolated form cultured astrocytes, suggesting that the stimulation of glycolytic flux by 8-hydroxy-efavrienz is not caused by direct inhibition of respiration. The observed alteration of astrocytic glucose metabolism by 8-hydroxy-efavirenz could contribute to the adverse neurological side effects reported for patients that are chronically treated with efavirenz-containing medications.





Similar content being viewed by others
References
Tan IL, McArthur JC (2012) HIV-associated neurological disorders: a guide to pharmacotherapy. CNS Drugs 26:123–134
Mirza A, Rathore MH (2012) Human immunodeficiency virus and the central nervous system. Semin Pediatr Neurol 19:119–123
Bazzoli C, Jullien V, Le Tiec C, Rey E, Mentre F, Taburet AM (2010) Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin Pharmacokinet 49:17–45
Volberding PA, Deeks SG (2010) Antiretroviral therapy and management of HIV infection. Lancet 376:49–62
Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Günthard HF, Hammer SM, Reiss P, Richman DD, Rizzsardini G, Thomas DL, Jacobsen DM, Volberding PA (2012) Antiretroviral treatment of adult HIV infection 2010 Recommentationsof the international AIDS society-USA panel. JAMA 304:321–333
Liner KJ 2nd, Ro MJ, Robertson KR (2010) HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep 7:85–91
Mothobi NZ, Brew BJ (2012) Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis 25:4–9
Brandmann M, Tulpule K, Schmidt MM, Dringen R (2012) The antiretroviral protease inhibitors indinavir and nelfinavir stimulate Mrp1-mediated GSH export from cultured brain astrocytes. J Neurochem 120:78–92
Arend C, Brandmann M, Dringen R (2013) The antiretroviral protease inhibitor ritonavir accelerates glutathione export from cultured primary astrocytes. Neurochem Res 38:732–741
Bumpus NN (2011) Efavirenz and 8-hydroxyefavirenz induce cell death via a JNK- and BimEL-dependent mechanism in primary human hepatocytes. Toxicol Appl Pharmacol 257:227–234
Avery LB, VanAusdall JL, Hendrix CW, Bumpus NN (2013) Compartmentalization and antiviral effect of efavirenz metabolites in blood plasma, seminal plasma, and cerebrospinal fluid. Drug Metab Dispos 41:422–429
De Clercq E (2004) Antiviral drugs in current clinical use. J Clin Virol 30:115–133
Jena A, Sachdeva RK, Sharma A, Wanchu A (2009) Adverse drug reactions to nonnucleoside reverse transcriptase inhibitor-based antiretroviral regimen: a 24-week prospective study. J Int Assoc Phys AIDS Care (Chic) 8:318–322
Lochet P, Peyriere H, Lotthe A, Mauboussin JM, Delmas B, Reynes J (2003) Long-term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Med 4:62–66
Fumaz CR, Munoz-Moreno JA, Molto J, Negredo E, Ferrer MJ, Sirera G, Perez-Alvarez N, Gomez G, Burger D, Clotet B (2005) Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr 38:560–565
Cespedes MS, Aberg JA (2006) Neuropsychiatric complications of antiretroviral therapy. Drug Saf 29:865–874
Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. Aids 15:71–75
Streck EL, Ferreira GK, Scaini G, Rezin GT, Goncalves CL, Jeremias IC, Zugno AI, Ferreira GC, Moreira J, Fochesato CM, Romao PR (2011) Non-nucleoside reverse transcriptase inhibitors efavirenz and nevirapine inhibit cytochrome C oxidase in mouse brain regions. Neurochem Res 36:962–966
Streck EL, Scaini G, Rezin GT, Moreira J, Fochesato CM, Romao PRT (2008) Effects of the HIV treatment drugs nevirapine and efavirenz on brain creatine kinase activity. Metab Brain Dis 23:485–492
Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z (2010) Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos 38:1218–1229
Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, Christ DD (1999) Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos 27:1319–1333
Ngaimisi E, Mugusi S, Minzi OM, Sasi P, Riedel KD, Suda A, Ueda N, Janabi M, Mugusi F, Haefeli WE, Burhenne J, Aklillu E (2010) Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther 88:676–684
Tovar-y-Romo LB, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, McArthur JC, Haughey NJ (2012) Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther 343:696–703
Dienel GA (2013) Astrocytic energetics during excitatory neurotransmission: what are contributions of glutamate oxidation and glycolysis? Neurochem Int 63:244–258
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63:177–188
Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27
Hamprecht B, Löffler F (1985) Primary glial cultures as a model for studying hormone action. Methods Enzymol 109:341–345
Tulpule K, Hohnholt M, Hirrlinger J, Dringen R (2014) Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In: Waagepetersen H, Hirrlinger J (eds) Neuromethods: Brain Energy Metabolism (in press)
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Brain Res Protoc 2:223–228
Scheiber IF, Schmidt MM, Dringen R (2010) Zinc prevents the copper-induced damage of cultured astrocytes. Neurochem Int 57:314–322
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein Measurement with the Folin Phenol Reagent. J Biol Chem 193:265–275
Minich T, Yokota S, Dringen R (2003) Cytosolic and mitochondrial isoforms of NADP+-dependent isocitrate dehydrogenases are expressed in cultured rat neurons, astrocytes, oligodendrocytes and microglial cells. J Neurochem 86:605–614
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Liddell JR, Zwingmann C, Schmidt MM, Thiessen A, Leibfritz D, Robinson SR, Dringen R (2009) Sustained hydrogen peroxide stress decreases lactate production by cultured astrocytes. Neurosci Res 87:2696–2708
Schmidt MM, Dringen R (2009) Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front Neuroenergetics 1:1–10
Blas-Garcia A, Apostolova N, Ballesteros D, Monleon D, Morales JM, Rocha M, Victor VM, Esplugues JV (2010) Inhibition of mitochondrial function by efavirenz increases lipid content in hepatic cells. Hepatology 52:115–125
Scheiber IF, Dringen R (2011) Copper accelerates glycolytic flux in cultured astrocytes. Neurochem Res 36:894–903
Tulpule K, Dringen R (2012) Formate generated by cellular oxidation of formaldehyde accelerates the glycolytic flux in cultured astrocytes. Glia 60:582–593
Chow YW, Leong CL, Chow HL, Hooi LS (2007) Lactic acidosis in HIV patients receiving highly active antiretroviral therapy. Med J Malaysia 62:78–80
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R (2011) Accumulation of PVP- coated silver nanoparticles by cultured astrocytes. Nanotechnology 22:375101
Heaton RK, Franklin, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Robertson K, Liner J, Meeker RB (2012) Antiretroviral neurotoxicity. J Neurovirol 18:388–399
Pauwels PJ, Opperdoes FR, Trouet A (1985) Effects of antimycin, glucose deprivation, and serum on cultures of neurons, astrocytes, and neuroblastoma cells. J Neurochem 44:143–148
Boffito M, Pillay D, Wilkins E (2006) Management of advanced HIV disease: resistance, antiretroviral brain penetration and malignancies. Int J Clin Pract 60:1098–1106
Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB, Collier AC, Gelman BB, Mbeo G, McCutchan JA, Simpson DM, Haubrich R, Ellis R, Grant I, Group C (2011) Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother 66:354–357
Goicoechea M, Best B (2007) Efavirenz/emtricitabine/tenofovir disoproxil fumarate fixed-dose combination: first-line therapy for all? Expert Opin Pharmacother 8:371–382
Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z (2003) The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 306:287–300
Kaddoumi A, Choi SU, Kinman L, Whittington D, Tsai CC, Ho RJY, Anderson BD, Unadkat JD (2007) Inhibition of P-glycoprotein activity at the primate blood-brain barrier increases the distribution of Nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos 35:1459–1462
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166
Goldman SA, Pulsinelli WA, Clarke WY, Kraig RP, Plum F (1989) The effects of extracellular acidosis on neurons and glia in vitro. J Cereb Blood Flow Metab 9:471–477
Testai FD, Gorelick PB (2010) Inherited metabolic disorders and stroke part 1: Fabry disease and mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Arch Neurol 67:19–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandmann, M., Nehls, U. & Dringen, R. 8-Hydroxy-efavirenz, the Primary Metabolite of the Antiretroviral Drug Efavirenz, Stimulates the Glycolytic Flux in Cultured Rat Astrocytes. Neurochem Res 38, 2524–2534 (2013). https://doi.org/10.1007/s11064-013-1165-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1165-2