Abstract
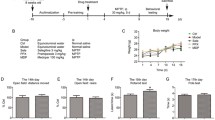
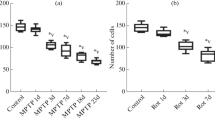
The tree shrew, a new experimental animal model, has been used to study a variety of diseases, especially diseases of the nervous system. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is the gold standard for toxin-based animal models of Parkinson’s disease (PD) because MPTP treatment replicates almost all of the pathological hallmarks of PD. Therefore, in this study, the effects of MPTP on the motor function of the tree shrew were examined. After five daily injections of a 3 mg/kg dose of MPTP, the motor function of MPTP-injected tree shrews decreased significantly, and the classic Parkinsonian symptoms of action and resting tremor, bradykinesia, posture abnormalities, and gait instability were observed in most MPTP-injected tree shrews. HPLC results also showed significantly reduced striatal dopamine and 3,4-dihydroxyphenylacetic acid levels in tree shrews after MPTP injection. Increased oxidative stress levels are usually considered to be the cause of dopaminergic neuron depletion in the presence of MPTP and were observed in the substantia nigra of MPTP-treated tree shrews, as indicated by a significant reduction in superoxide dismutase and glutathione peroxidase activity and increased levels of malondialdehyde. In addition, elevated α-synuclein mRNA levels in the midbrain of MPTP-treated tree shrews were observed. Furthermore, MPTP-treated tree shrews showed the classic Parkinsonian symptoms at a lower MPTP dosage compared with other animal models. Thus, the MPTP-treated tree shrew may be a potential animal model for studying the pathogenesis of PD.




Similar content being viewed by others
References
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol 5:677–687
Geng X, Tian X, Tu P, Pu X (2007) Neuroprotective effects of echinacoside in the mouse MPTP model of Parkinson’s disease. Eur J Pharmacol 564:66–74
Heikkila RE, Hess A, Duvoisin RC (1984) Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 224:1451–1453
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol 2012:845618
Bartolomucci A, de Biurrun G, Fuchs E (2001) How tree shrews (Tupaia belangeri) perform in a searching task: evidence for strategy use. J Comp Psychol 115:344–350
Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E (2001) Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA 98:12796–12801
Fuchs E (2005) Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr 10:182–190
Fuchs E, Flugge G (2002) Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol Biochem Behav 73:247–258
Fuchs E, Uno H, Flugge G (1995) Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Res 673:275–282
Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498
McCoy P, Norton TT, McMahon LL (2008) Layer 2/3 synapses in monocular and binocular regions of tree shrew visual cortex express mAChR-dependent long-term depression and long-term potentiation. J Neurophysiol 100:336–345
Vezoli J, Fifel K, Leviel V, Dehay C, Kennedy H, Cooper HM, Gronfier C, Procyk E (2011) Early presymptomatic and long-term changes of rest activity cycles and cognitive behavior in a MPTP-monkey model of Parkinson’s disease. PLoS One 6:e23952
Chiba-Falek O, Lopez GJ, Nussbaum RL (2006) Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord 21:1703–1708
Lo YC, Shih YT, Tseng YT, Hsu HT (2012) Neuroprotective effects of San-Huang-Xie-Xin-Tang in the MPP(+)/MPTP Models of Parkinson’s disease in vitro and in vivo. Evid Based Complement Alternat Med 2012:501032
Li XZ, Chen XP, Zhao K, Bai LM, Zhang H, Zhou X (2012) Therapeutic effects of valproate combined with lithium carbonate on MPTP-induced Parkinsonism in mice: possible mediation through enhanced autophagy. Int J Neurosci (PubMed: 22978383)
Wu DD, Huang L, Zhang L, Wu LY, Li YC, Feng L (2012) LLDT-67 attenuates MPTP-induced neurotoxicity in mice by up-regulating NGF expression. Acta Pharmacol Sin 33:1187–1194
Villalba RM, Smith Y (2011) Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J Comp Neurol 519:989–1005
Javitch JA, Snyder SH (1984) Uptake of MPP(+) by dopamine neurons explains selectivity of parkinsonism-inducing neurotoxin, MPTP. Eur J Pharmacol 106:455–456
Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36:2503–2508
Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL (1998) The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods 83:165–175
Haobam R, Sindhu KM, Chandra G, Mohanakumar KP (2005) Swim-test as a function of motor impairment in MPTP model of Parkinson’s disease: a comparative study in two mouse strains. Behav Brain Res 163:159–167
Liebetanz D, Baier PC, Paulus W, Meuer K, Bahr M, Weishaupt JH (2007) A highly sensitive automated complex running wheel test to detect latent motor deficits in the mouse MPTP model of Parkinson’s disease. Exp Neurol 205:207–213
Bezard E, Imbert C, Deloire X, Bioulac B, Gross CE (1997) A chronic MPTP model reproducing the slow evolution of Parkinson’s disease: evolution of motor symptoms in the monkey. Brain Res 766:107–112
Politis M, Wu K, Molloy S, Bain PG, Chaudhuri KR, Piccini P (2010) Parkinson’s disease symptoms: the patient’s perspective. Mov Disord 25:1646–1651
Sowell RA, Owen JB, Butterfield DA (2009) Proteomics in animal models of Alzheimer’s and Parkinson’s diseases. Ageing Res Rev 8:1–17
Madras BK (1994) 11C-WIN 35,428 for detecting dopamine depletion in mild Parkinson’s disease. Ann Neurol 35:376–377
Bloem BR, Irwin I, Buruma OJ, Haan J, Roos RA, Tetrud JW, Langston JW (1990) The MPTP model: versatile contributions to the treatment of idiopathic Parkinson’s disease. J Neurol Sci 97:273–293
Ferraro TN, Golden GT, DeMattei M, Hare TA, Fariello RG (1986) Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on levels of glutathione in the extrapyramidal system of the mouse. Neuropharmacology 25:1071–1074
Simonian NA, Coyle JT (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47:S161–S170
Graham DG (1979) On the origin and significance of neuromelanin. Arch Pathol Lab Med 103:359–362
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313:324–328
Mori F, Nishie M, Kakita A, Yoshimoto M, Takahashi H, Wakabayashi K (2006) Relationship among alpha-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. J Neuropathol Exp Neurol 65:808–815
Acknowledgments
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20121106120056), Yunnan Natural Science Foundation (No. 2013FZ132; No. 2011FB116), National Natural Science Foundation (No. 31100127) and the National Science and Technology Support Project (No. 2009BAI83B02-21; No. 2011BAI15B01-21; No. 2012BAI39B01).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 32617 kb)
Supplementary material 2 (MP4 6310 kb)
Rights and permissions
About this article
Cite this article
Ma, KL., Gao, JH., Huang, ZQ. et al. Motor Function in MPTP-Treated Tree Shrews (Tupaia belangeri chinensis). Neurochem Res 38, 1935–1940 (2013). https://doi.org/10.1007/s11064-013-1099-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1099-8