Abstract
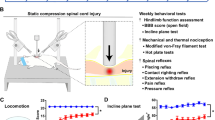
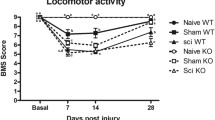
Chronic neuropathic pain is a disabling condition observed in large number of individuals following spinal cord injury (SCI). Recent progress points to an important role of neuroinflammation in the pathogenesis of central neuropathic pain. The focus of the present study is to investigate the role of proinflammatory molecules IL-1β, TNF-α, MCP-1, MMP-9 and TIMP-1 in chronic neuropathic pain in a rodent model of SCI. Rats were subjected to spinal cord contusion using a controlled linear motor device with an injury epicenter at T10. The SCI rats had severe impairment in locomotor function at 7 days post-injury as assessed by the BBB score. The locomotor scores showed significant improvement starting at day 14 and thereafter showed no further improvement. The Hargreaves’ test was used to assess thermal hyperalgesia for hindpaw, forepaw and tail. A significant reduction in withdrawal latency was observed for forepaw and tail of SCI rats at days 21 and 28, indicating the appearance of thermal hyperalgesia. Changes in expression of mRNAs for IL-1β, TNF-α, MCP-1, MMP-9 and TIMP-1 were assessed using real-time polymerase chain reaction in spinal cord including the injury epicenter along with regions above and below the level of lesion at day 28 post-injury. A significant increase was observed in the expression of MCP-1, TNF-α, TIMP-1 and IL-1β in the injury epicenter, whereas only TIMP-1 was upregulated in the area below the injury epicenter. The results of the study suggest that prolonged upregulation of inflammatory mediators might be involved in chronic neuropathic pain in SCI, and that TIMP-1 may play a role in maintenance of chronic below level pain.




Similar content being viewed by others
Abbreviations
- IL-1β:
-
Interleukin 1 beta
- MCP-1:
-
Monocyte chemotactic protein-1
- MMP-9:
-
Matrix metalloproteinase 9
- TIMP-1:
-
Tissue inhibitor of metalloproteinase 1
- TNF-α:
-
Tumor necrosis factor-alpha
References
Sekhon LHS, Fehlings MG (2001) Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 26(24S):S2–S12
Deumens R, Joosten EA, Waxman SG, Hains BC (2008) Locomotor dysfunction and pain: the scylla and charybdis of fiber sprouting after spinal cord injury. Mol Neurobiol 37:52–63
Judith AT, Diana DC, Catherine AW, Catherine BM (2001) Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil 82:501–509
Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS (2001) Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord 39:256–262
Gwak YS, Crown ED, Unabia GC, Hulsebosch CE (2008) Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain 138:410–422
Anderson KD (2004) Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21:1371–1383
Woolf CJ, Mannion RJ (1999) Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353:1959–1964
Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ et al (2003) Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 60:1524–1534
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10:23–36
Moalem G, Tracey DJ (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 51:240–264
Hulsebosch CE (2008) Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol 214:6–9
Chang HT (2007) Subacute human spinal cord contusion: few lymphocytes and many macrophages. Spinal Cord 45:174–182
Zhao P, Waxman SG, Hains BC (2007) Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci 27:2357–2368
McKay SM, Brooks DJ, Hu P, McLachlan EM (2007) Distinct types of microglial activation in white and grey matter of rat lumbosacral cord after mid-thoracic spinal transection. J Neuropathol Exp Neurol 66:698–710
Zhao P, Waxman SG, Hains BC (2007) Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27:8893–8902
Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC et al (2007) Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci 27:6006–6018
Mika J (2008) Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep 60:297–307
Chattopadhyay S, Myers RR, Janes J, Shubayev V (2007) Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain, Behavior, Immun 21:561–568
Alexander JK, Popovich PG (2009) Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res 175:125–137
Tan AM, Zhao P, Waxman SG, Hains BC (2009) Early microglial inhibition preemptively mitigates chronic pain development after experimental spinal cord injury. J Rehabil Res Dev 46:123–133
Ramu J, Bockhorst KH, Mogatadakala KV, Narayana PA (2006) Functional magnetic resonance imaging in rodents: methodology and application to spinal cord injury. J Neurosci Res 84:1235–1244
Bilgen M (2005) A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair 19:219–226
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Yamamotova A, Sramkova T, Rokyta R (2010) Intensity of pain and biochemical changes in blood plasma in spinal cord trauma. Spinal Cord 48:21–26
Gwak YS, Hains BC, Johnson KM, Hulsebosch CE (2004) Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat. Neurosci Lett 362:232–235
Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM (2008) Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 212:337–347
Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA et al (2007) Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 149:706–714
Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S (2007) Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 27:12396–12406
Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ et al (2009) JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 29:4096–4108
Liu SQ, Ma YG, Peng H, Fan L (2005) Monocyte chemoattractant protein-1 level in serum of patients with acute spinal cord injury. Chin J Traumatol 8:216–219
Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C et al (2007) Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain 3:38
Knerlich-Lukoschus F, Juraschek M, Blomer U, Lucius R, Mehdorn HM, Held-Feindt J (2008) Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma 25:427–448
White FA, Feldman P, Miller RJ (2009) Chemokine signaling and the management of neuropathic pain. Mol Interv 9:188–195
Ji R-R, Xu Z-Z, Wang X, Lo EH (2009) Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci 30:336–340
Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH et al (2008) Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 14:331–336
Goussev S, Hsu JY, Lin Y, Tjoa T, Maida N, Werb Z et al (2003) Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J Neurosurg 99:188–197
Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K et al (2009) Neuroprotective effects of over-expressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma 26:1935–1941
Stetler-Stevenson WG (2008) Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1:re6
Chirco R, Liu XW, Jung KK, Kim HR (2006) Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25:99–113
Davies AL, Hayes KC, Dekaban GA (2007) Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 88:1384–1393
Nakagawa T (2010) Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci 114:347–353
Acknowledgments
The financial assistance provided by the Steve Palermo Endowment and the National Institute of Health (P30-HD02528) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandhir, R., Gregory, E., He, YY. et al. Upregulation of Inflammatory Mediators in a Model of Chronic Pain after Spinal Cord Injury. Neurochem Res 36, 856–862 (2011). https://doi.org/10.1007/s11064-011-0414-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0414-5