Abstract
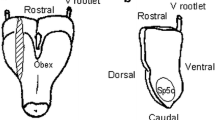
This study was designed to shed more light onto the three different brainstem regions which are implicated in the pain pathway for the level of various excitatory and inhibitory neurotransmitters before and following neuronal stimulation. The in vivo microdialysis technique was used in awake, freely moving adult Sprague-Dawley rats. The neurotransmitters studied included aspartate, glutamate, GABA, glycine, and taurine. The three brainstem regions examined included the mid-brain periaqueductal gray (PAG), the medullary nucleus raphe magnus (NRM), and the spinal trigeminal nucleus (STN). Neuronal stimulation was achieved following the administration of the sodium channel activator veratridine. The highest baseline levels of glutamate (P < 0.0001), aspartate (P < 0.0001), GABA (P < 0.01), taurine (P < 0.0001), and glycine (P < 0.001) were seen in the NRM. On the other hand, the lowest baseline levels of glutamate, GABA, glycine, and taurine were found in the PAG, while that of aspartate was found in the STN. Following the administration of veratridine, the highest release of the above neurotransmitters except for the aspartate and glycine was found in the PAG where the level of glutamate increased by 1,310 ± 293% (P < 0.001), taurine by 1,008 ± 143% (P < 0.01), and GABA by 10,358 ± 1,920% (P < 0.0001) when comparison was performed among the three brainstem regions and in relation to the baseline levels. The highest release of aspartate was seen in the STN (2,357 ± 1,060%, P < 0.001), while no significant difference was associated with glycine. On the other hand, the lowest release of GABA and taurine was found in the STN (696 ± 91 and 305 ± 25%, respectively), and glutamate and aspartate in the NRM (558 ± 200 and 874 ± 315%, respectively). Our results indicate, and for the first time, that although some differences are seen in the baseline levels of the above neurotransmitters in the three regions studied, there are quite striking variations in the level of release of these neurotransmitters following neuronal stimulation in these regions. In our opinion this is the first study to describe the pain activation/modulation related changes of the excitatory and inhibitory amino acids profile of the three different brainstem areas.





Similar content being viewed by others
References
Basbaum AI, Fields HL (1984) The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 187:513–532
Renn CL, Dorsey SG (2005) The physiology and processing of pain: a review. AACN Clin Issues 16:277–290
Fang FG, Haws CM, Drasner K, Williamson A, Fields HL (1989) Opioid peptides (DAGO-enkephalin, dynorphin A(1–13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res 501:116–128
Renno WM, Mullett MA, Beitz AJ (1992) Systemic morphine reduces GABA release in the lateral but not the medial portion of the midbrain periaqueductal gray of the rat. Brain Res 594:221–232
Bennet GJ, Mayer DJ (1979) Inhibition of spinal cord interneurons by narcotic injections. Brain Res 172:243–257
Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164:444–445
Holstege G, Kuypers HGJM (1982) The anatomy of brainstem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res 57:45–75
Stanford JA (1995) Descending control of pain. Br J Anaesth 75:217–227
Fields HL, Hienricher MM, Mason P (1991) Neurotransmitters in nociceptive modulatory circuits. Anal Rev Neurosci 14:219–245
Keay KA, Bandler R (1993) Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci Lett 154:23–26
Silva E, Hernandez L, Contreras Q, Guerrero F, Alba G (2000) Noxious stimulation increases glutamate and arginine in the periaqueductal gray matter in rats: a microdialysis study. Pain 87:131–135
Maione S, Marabese I, Oliva P, de Novellis V, Stella L, Rossi F, Filippelli A (1999) Periaqueductal gray matter glutamate and GABA decrease following subcutaneous formalin injection in rat. Neuroreport 10:1403–1407
Renno WM (1998) Microdialysis of excitatory amino acids in the periaqueductal gray of the rat after unilateral peripheral inflammation. Amino Acids 4:319–331
Renno WM, Mullett MA, Willims FG, Beitz AJ (1998) Construction of 1MM microdialysis probe for amino acids dialysis in rats. J Neurosci Methods 79:217–228
Benveniste H, Huttemeier PC (1990) Microdialysis: theory and application. Prog Neurobiol 35:195–215
Di Chiara G (1990) In vivo brain dialysis of neurotransmitters. Trends Pharmacol Sci 11:116–121
Renno WM, Beitz AJ (1999) Peripheral inflammation is associated with decrease veratridine-induced release of GABA in the rat ventrocaudal periaqueductal gray: microdialysis study. J Neurol Sci 163:105–110
Beck H (2007) Plasticity of antiepileptic drug targets. Epilepsia 48(Suppl 1):14–18
Rogawski MA, Loscher W (2004) The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 10(7):685–692
Zaremba PD, Bialek M, Blaszczyk B, Cioczek P, Czuczwar SJ (2006) Non-epilepsy uses of antiepilepsy drugs. Pharmacol Rep 58(1):1–12
Paxinos G, Watson C (1986) The rat brain in stereotoxic coordinates, 2nd edn. Academic, Australia
Lindorth O, Mopper K (1974) High performance liquid chromatographic determination in picomole amounts of amino acids by precolumn fluorescence derivatization with 0-phthaldialdehyde. Anal Chem 51:1667–1674
Beitz AJ (1990) Relationship of glutamate and aspartate to the periaqueductal gray raphe magnus projection: analysis using immuno cytochemistry and microdialysis. J Histochem Cytochem 38:1755–1765
Skilling SR, Mullett M, Beitz AJ, Larson AA (1992) Fluorogold administration via microdialysis labels neurons terminating with the dialysis region. J Neurosci Methods 41:85–90
Jacquet YF (1988) The NMDA receptor: central role in pain inhibition in the rat periaqueductal gray. Eur J Pharmacol 154:271–276
Goodchild AK, Dampney RAL, Bandler RA (1928) A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of central nervous system. J Neurosci Methods 6:351–363
Aimone LD, Gebhart GF (1986) Stimulation-produced spinal inhibition from the mid-brain in the rate is mediated by an excitatory amino acid neurotransmitter in the medial medulla. J Neurosci 7:1803–1813
Van Praag H, Frenk H (1990) The role of glutamate in opiate descending inhibition of nociceptive spinal reflexes. Brian Res 524:101–105
Gold MS, Morgan MM, Liebeskind JC (1990) Antinociceptive and behavioral effects of low dose kainic acid injections into the PAG of the rat. Pain Suppl 5:441
Clements JR, Madl JE, Johnson RL, Larson AA, Beitz AJ (1987) Localization of glutamate, glutaminase, aspartate and aspartate aminotransferase in the rat midbrain periaqueductal gray. Exp Brain Res 67:594–602
Albin RL, Makowiec RL, Hollingsworth Z, Dure LS IV, Penney JB, Young AB (1990) Excitatory amino acid binding sites in the periaqueductal gray of the rat. Neurosci Lett 118(1):112–115
Cotman CW, Monaghan DT, Ottersen OP, Storm-Mathisen J (1987) Anatomical excitatory amino acid receptors and their pathways. Trends Neurosci 10:273–280
Reichling DB, Basbaum AI (1990) Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: II. Electron microscopic immunocytochemical evidence, of GABAergic control over the projection from the periaqueductal gray to the nucleus raphe magnus in the rat. J Comp Neurol 302:378–393
Cho HJ, Basbaum AI (1991) GABAergic circuitry in the rostral ventral medulla of the rat and its relationship to descending antinociceptive controls. J Comp Neurol 303:316–328
Moreau JL, Fields HL (1986) Evidence for GABA involvement in midbrain control of meullary neurons that moduclate noceiceptive transmission. Brain Res 397:37–46
Drower EJ, Hammond DL (1988) GABAergic modulation of noviceptive threshold: effect of THIP and bicucculine microinjected in the ventral medulla of the rat. Brain Res 450:316–324
Fields HL, Anderson SD (1991) Evidence that raphe-spinal neurons mediate opiate and midbrain stimulation-produced analgesia. Pain 5:333–349
Morgan MM, Clayton CC, Lane DA (2003) Behavioral evidence linking opioid-sensitive GABAergic neurons in the ventrolateral periaqueductal gray to morphine tolerance. Neuroscience 118:227–232
de Novellis V, Marabese I, Palazzo E, Rossi F, Berrino L, Rodella L, Bianchi R, Rossi F, Maione S (2003) Group I metabotropic glutamate receptors modulate glutamate and gamma-aminobutyric acid release in the periaqueductal grey of rats. Eur J Pharma 462:73–81
Huxtable RJ (1989) Taurine in the central nervous system and the mammalian actions of taurine. Prog Neurobiol 32:471–533
Palkovits M, Banny-Schwartz M, Lijtha A (1990) Taurine levels in brain nuclei of your adult and agin rats. In: Pasantes-Marates H, Marin DL, Shain W, Del Rio RM (eds) Taurine: functional neurochemistrey, physiology and cardiology, Wiley-Liss, New York. Prog Cli. Biol Res 351:1333–1335
Lerma J, herranz AS, herreras O, Abraira V, Martin Del Rio R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 84:145–155
Vanegas H (2004) To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett 361:225–228
Wei F, Ren K, Dubner R (1998) Inflammation-induced Fos protein expression in the rat spinal cord is enhanced following dorsolateral or ventrolateral funiculus lesions. Brain Res 782:136–141
Wei F, Dubner R, Ren K (1999) Nucleus reticularis gigantocellularis and nucleus raphe magnus in the brain stem exert opposite effects on behavioral hyperalgesia and spinal Fos protein expression after peripheral inflammation. Pain 80:127–141; Erratum Pain 81:215–219
Sandkühler J, Eblen-Zajjur A, Fu QG, Forster C (1995) Differential effects of spinalization on discharge patterns and discharge rates of simultaneously recorded nociceptive and non-nociceptive spinal dorsal horn neurons. Pain 60:55–65
Bereiter DA, Benetti AP (1996) Excitatory amino release within spinal trigeminal nucleus after mustard oil injection into the temporomandibular joint region of the rat. Pain 67:451–459
Elekes I, Patthy A, Lang T, Palkovits M (1989) Concentrations of GABA and glycine in discrete brain nuclei. Stress-induced changes in the levels of inhibitory amino acids. Neuropharmacology 25:703–709
Ljungdahl A, Hiikfelt T (1873) Autoradiographic uptake patterns of (3H)GABA and (3H)glycine in central nervous tissues with special reference to the cat spinal cord. Brain Res 62:587–595
Storm-Mathisen J, Ottersen OP, Fu-Long T, Gundersen V, Laake JH, Nordbo G (1986) Metabolism and transport of amino acids studied by immunocytochemistry. Med Biol 64(2–3):127–132
Clements JR, Magnusson KR, Beitz AJ (1990) Ultrastructural description of glutamate, aspartate, taurine, and glycine-like immunoreactive terminals from five rat brain regions. J Electron Microsc Tech 15:49–66
Pourcho RG, Goebel DJ, Jojich L, Hazlett JC (1992) Immunocytochemical evidence for the involvement of glycine in sensory centers of the rat brain. Neuroscience 46:643–656
Li YQ, Takada M, Kaneko T, Mizuno N (1996) GABAergic and glycinergic neurons projecting to the trigeminal motor nucleus: a double labeling study in the rat. J Comp Neurol 373:498–510
Todd AJ, Watt C, Spike RC, Sieghart W (1996) Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. Neuroscience 16:974–982
Wang D, Wu JH, Dong YX, Li YQ (2001) Synaptic connections between trigemino-parabrachial projection neurons and gamma-aminobutyric acid- and glycine-immunoreactive terminals in the rat. Brain Res 921:133–137
Cortes R, Palacios JM (1990) Autoradiographic mapping fo glycine receptors by [3H] strychnine binding. In: Otterson OP, Storm-Mathisen J (eds) Glycine neurotransmission. Wiley, UK, pp 2239–2263
Sato K, Zhang JH, Saika T, Sato M, Tada K, Tohyama M (1990) Localization of glycine receptor alpha 1 subunit mRNA-containing neurons in the rat brain: an analysis using in situ hybridization histochemistry. Neuroscience 43:381–395
Acknowledgments
The authors would like to thank Mrs. Solly Alex and Aksa Mathew for their technical assistance. We would like also to thank Dr. Ghanim Alkhaledi for reviewing our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renno, W.M., Alkhalaf, M., Mousa, A. et al. A Comparative Study of Excitatory and Inhibitory Amino Acids in Three Different Brainstem Nuclei. Neurochem Res 33, 150–159 (2008). https://doi.org/10.1007/s11064-007-9427-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9427-5