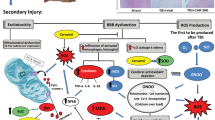
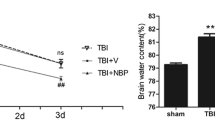
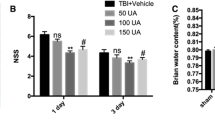
Abstract
N-acetylcysteine (NAC) is a precursor of glutathione, a potent antioxidant, and a free radical scavenger. The beneficial effect of NAC on nervous system ischemia and ischemia/reperfusion models has been well documented. However, the effect of NAC on nervous system trauma remains less understood. Therefore, we aimed to investigate the therapeutic efficacy of NAC with an experimental closed head trauma model in rats. Thirty-six adult male Sprague–Dawley rats were randomly divided into three groups of 12 rats each: Group I (control), Group II (trauma-alone), and Group III (trauma+NAC treatment). In Groups II and III, a cranial impact was delivered to the skull from a height of 7 cm at a point just in front of the coronal suture and over the right hemisphere. Rats were sacrificed at 2 h (Subgroups I-A, II-A, and III-A) and 12 h (Subgroups I-B, II-B, and III-B) after the onset of injury. Brain tissues were removed for biochemical and histopathological investigation. The closed head trauma significantly increased tissue malondialdehyde (MDA) levels (P<0.05), and significantly decreased tissue superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities (P<0.05), but not tissue catalase (CAT) activity, when compared with controls. The administration of a single dose of NAC (150 mg/kg) 15 min after the trauma has shown protective effect via decreasing significantly the elevated MDA levels (P<0.05) and also significantly (P<0.05) increasing the reduced antioxidant enzyme (SOD and GPx) activities, except CAT activity. In the trauma-alone group, the neurons became extensively dark and degenerated into picnotic nuclei. The morphology of neurons in the NAC treatment group was well protected. The number of neurons in the trauma-alone group was significantly less than that of both the control and trauma+NAC treatment groups. In conclusion, the NAC treatment might be beneficial in preventing trauma-induced oxidative brain tissue damage, thus showing potential for clinical implications.


Similar content being viewed by others
References
Siesjo BK (1993) Basic mechanisms of traumatic brain damage. Ann Emerg Med 22:959–969
Marshall LF (2000) Head injury: recent past, present, and future. Neurosurgery 47:546–561
Ozsuer H, Gorgulu A, Kiris T, Cobanoglu S (2005) The effects of memantine on lipid peroxidation following closed-head trauma in rats. Neurosurg Rev 28:143–147
Ellis EF, Dodson LY, Police RJ (1991) Restoration of cerebrovascular responsiveness to hyperventilation by the oxygen radical scavenger N-acetylcysteine following experimental traumatic brain injury. J Neurosurg 75:774–749
Sastry PS, Rao KS (2000) Apoptosis and the nervous system. J Neurochem 74:1–20
Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK (1998) Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J Neurosci 18:5663–5672
Kaya SS, Mahmood A, Li Y, Yavuz E, Goksel M, Chopp M (1999) Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Res 818:23–33
Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK (1995) Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol 47:1575–1583
Charriaut-Marlangue C (1999) Apoptosis and cerebral ischemia. Ann Pharm Fr 57:309–313
Michel PP, Lambeng N, Ruberg M (1999) Neuropharmacologic aspects of apoptosis: significance for neurodegenerative diseases. Clin Neuropharmacol 22:137–150
Mirabella M, Di Giovanni S, Silvestri G, Tonali P, Servidei S (2000) Apoptosis in mitochondrial encephalomyopathies with mitochondrial DNA mutations: a potential pathogenic mechanism. Brain 123:93–104
Villa P, Kaufmann SH, Earnshaw WC (1997) Caspases and caspase inhibitors. Trends Biochem Sci 22:388–393
Allen JW, Knoblach SM, Faden AI (1999) Combined mechanical trauma and metabolic impairment in vitro induces NMDA receptor-dependent neuronal cell death and caspase-3 dependent apoptosis. FASEB J 13:1875–1882
Yakovlev AG, Knoblach SM, Fan L, Fox GB, Goodnight R, Faden AI (1997) Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J Neurosci 17:7415–7424
Clark RS, Kochanek PM, Watkins SC, Chen M, Dixon CE, Seidberg NA, Melick J, Loeffert JE, Nathaniel PD, Jin KL, Graham SH (2000) Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem 74:740–753
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
McCall JM, Braughler JM, Hall ED (1987) Lipide peroxidation and the role of oxygen radicals in CNS injury. Acta Anaesthesiol Belg 38:373–379
Ikeda Y, Long DM (1990) The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery 27:1–11
Clausen F, Lundqvist H, Ekmark S, Lewen A, Ebendal T, Hillered L (2004) Oxygen free radical-dependent activation of extracellular signal-regulated kinase mediates apoptosis-like cell death after traumatic brain injury. J Neurotrauma 21:1168–1182
Faden AI (1989) TRH analogYM-14673 improves outcome following traumatic brain and spinal cord injury in rats: dose response studies. Brain Res 486:228–235
Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant effect of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597
Santangelo F (2003) Intracellular thiol concentration modulating inflammatory response: influence on the regulation of cell functions through cysteine prodrug approach. Curr Med Chem 10:2599–2610
Warner DS (2004) Oxidants, antioxidants and the ischemic brain. J Exp Biol 207: 3221–3231
Krasowska A, Konat GW (2004) Vulnerability of brain tissue to inflammatory oxidant, hypochlorous acid. Brain Res 997:176–184
Demir S, Inal-Erden M (1998) Pentoxifylline and N-acetylcysteine in hepatic ischemia/reperfusion injury. Clin Chim Acta 275: 127–135
Cakir O, Erdem K, Oruc A, Kilinc N, Eren N (2003) Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovasc Surg 11: 375–379
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE (2003) The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84:1173–1183
Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK (2004) N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res 78:347–361
Hart AM, Terenghi G, Kellerth JO, Wiberg M (2004) Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience 125:91–101
Thomas Dickey D, Muldoon LL, Kraemer DF, Neuwelt EA (2004) Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear Res 193:25–30
Nicoletti VG, Marino VM, Cuppari C, Licciardello D, Patti D, Purello VS, Stella AM (2005) Effect of antioxidant diets on mitochondrial gene expression in rat brain during aging. Neurochem Res 30:737–752
Cuzzocrea S, Mazzon E, Costantino G, Serraino I, Dugo L, Calabro G, Cucinotta G, De Sarro A, Caputi AP (2000) Effects of N-acetylcysteine in a rat model of ischemia and reperfusion injury. Cardiovasc Res 47:537–548
Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK (2003) N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971:1–8
Shen WH, Zhang CY, Zhang GY (2003) Antioxidants attenuate reperfusion injury after global brain ischemia through inhibiting nuclear factor-kappa B activity in rats. Acta Pharmacol Sin 24:1125–1130
Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK (2004) Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res 76:519–527
Shapira Y, Shohami E, Sidi S, Soffer D, Freeman S, Cotev S (1988) Experimental closed head injury in rats: mechanical, pathophysiologic and neurologic properties. Crit Care Med 16:258–265
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid rection. Anal Biochem 95:351–358
Lowry OH, Rosebrough NJ, Farr AL, Randall RI (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Aebi H (1974) Catalase in vitro. Methods Enzymol 105:121–126
Hsu SM, Raine L, Fanger H (1981) Use of Avidin-Biotin-Peroxidase complex (ABC) in immunperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Sochman J (2002) N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know. J Am Coll Cardiol 39:1422–1428
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54:271–284
Smith SL, Andrus PK, Zhang JR, Hall ED (1994) Direct measurement of hydroxyl radicals, lipid peroxidation, and blood–brain barrier disruption following unilateral cortical impact head injury in the rat. J Neurotrauma 11:393–404
Hall ED (1995) Inhibition of lipid peroxidation in central nervous system trauma and ischemia. J Neurol Sci 134(Suppl):79–83
Thomale UW, Griebenow M, Kroppenstedt SN, Unterberg AW, Stover JF (2006) The effect of N-acetylcysteine on posttraumatic changes after controlled cortical impact in rats. Intensive Care Med 32:149–155
Ustun ME, Duman A, Ogun CO, Sumer F, Gubbilek M (2001) Effects of deferoxamine on tissue superoxide dismutase and glutathione peroxidase levels in experimental head trauma. J Trauma 51:22–25
Ozben T (1989) Mechanisms involved in neuronal damage. In: Ozben T (ed) Free radicals, oxidative stress and antioxidants. Plenum Press, New York, London, pp163–189
Jayalakshmi K, Sairam M, Singh SB, Sharma SK, Ilavazhagan G, Banerjee PK (2005) Neuroprotective effect of N-acetyl cysteine on hypoxia-induced oxidative stress in primary hippocampal culture. Brain Res 1046:97–104
Nehru B, Kanwar SS (2004) N-acetylcysteine exposure on lead-induced lipid peroxidative damage and oxidative defense system in brain regions of rats. Biol Trace Elem Res 101:257–264
Southorn PA (1988) Free radicals in medicine. Chemical Nature and biological reactions. Mayo Clin Proc 63:381–389
Ferrari G, Green LA (1995) N-acetylcysteine (d- and l-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci 15:2857–2866
Juurlink BH, Paterson PG (1998) Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med 21:309–334
Tripathi Y, Hegde BM, Rai YS, Raghuveer CV (1999) Effect of N-acetylcysteine and sodium nitrite on myocardial reperfusion injury. Indian J Physiol Pharmacol 43:518–520
Hashimoto K, Tsukada H, Nishiyama S, Fukumato D, Kakiuchi T, Shimizu E, Iyo M (2004) Protective effects of N-acetyl-l-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacol 29:2018–2023
Tian H, Zhang G, Li H, Zhang Q (2003) Antioxidant NAC and AMPA/KA receptor antagonist DNQX inhibited JNK3 activation following global ischemia in rat hippocampus. Neurosci Res 46:191–197
Kaynar MY, Erdincler P, Tadayyon E, Belce A, Gumustas K, Ciplak N (1998) Effect of nimodipine and N-acetylcysteine on lipid peroxidation after experimental spinal cord injury. Neurosurg Rev 21:260–264
Xiong Y, Peterson PL, Lee CP (1999) Effect of N-acetylcysteine on mitochondrial function following traumatic brain injury in rats. J Neurotrauma 16:1067–1082
Yi JK, Hazell AS (2005) N-acetylcysteine attenuates early induction of heme oxygenase-1 following traumatic brain injury. Brain Res 1033:13–19
Unterberg AW, Stroop R, Thomale UW, Kiening KL, Pauser S, Vollmann W (1997) Characterisation of brain edema following controlled cortical impact injury. Acta Neurochir Suppl 70:106–108
Yang X, Yang S, Zhang J, Xue L, Hu Z (2002) Role of Caspase-3 in neuronal apoptosis after acute brain injury. Chin J Traumatol 5:250–253
Lu J, Moochhala S, Shirhan M, Ng KC, Teo AL, Tan MH, Moore XL, Wong MC, Ling EA (2003) Neuroprotection by aminoguanidine after lateral fluid-percussive brain injury in rats: a combined magnetic resonance imaging, histopathologic and functional study. Neuropharmacology 44:253–263
Cernak I, Chapman SM, Hamlin GP, Vink R (2002) Temporal characterisation of pro- and anti-apoptotic mechanisms following diffuse traumatic brain injury in rats. J Clin Neurosci 9:565–572
Nicholson DW (1999) Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 6:1028–1042
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hicdonmez, T., Kanter, M., Tiryaki, M. et al. Neuroprotective Effects of N-acetylcysteine on Experimental Closed Head Trauma in Rats. Neurochem Res 31, 473–481 (2006). https://doi.org/10.1007/s11064-006-9040-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9040-z