Abstract
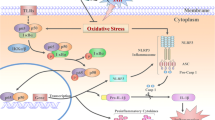
Early brain injury (EBI) is a major cause of mortality from subarachnoid hemorrhage (SAH). We aimed to study the pathophysiology of EBI and explore the role of hepcidin, a protein involved in iron homeostatic regulation, and its downstream proteins. One hundred and thirty-two male Sprague–Dawley rats were assigned into groups (n = 24/group): sham, SAH, SAH + hepcidin, SAH + hepcidin-targeting small interfering ribonucleic acid (siRNA), and SAH + scramble siRNA. Three hepcidin-targeting siRNAs and one scramble siRNA for hepcidin were injected 24 h before hemorrhage induction, and hepcidin protein was injected 30 min before induction. The rats were neurologically evaluated at 24 h and euthanized at 72 h. Hepcidin, ferroportin-1, and ceruloplasmin protein expression were measured by immunohistochemistry and Western blotting. Brain water content, blood–brain barrier (BBB) leakage, non-heme tissue iron and Garcia scale were evaluated. Hepcidin expression increased in the cerebral cortex and hippocampus after experimental SAH (P < 0.05 compared to sham), while ferroportin-1 and ceruloplasmin decreased (P < 0.05). Hepcidin injection lowered the expression of ferroportin-1 and ceruloplasmin further but siRNA reduced the levels of hepcidin (P < 0.05 compared to SAH) resulting in recovery of ferroportin-1 and ceruloplasmin levels. Apoptosis was increased in SAH rats compared to sham (P < 0.05) and increased slightly more by hepcidin, but decreased by siRNA (P < 0.05 compared to SAH). SAH rats had lower neurological scores, high brain water content, BBB permeability, and non-heme tissue iron (P < 0.05). In conclusion, downregulation of ferroportin-1 and ceruloplasmin caused by hepcidin enhanced iron-dependent oxidative damage and may be the potential mechanism of SAH.




Similar content being viewed by others
References
Cahill J, Zhang JH (2009) Subarachnoid hemorrhage: is it time for a new direction? Stroke 40(3 Suppl):S86–S87. doi:10.1161/strokeaha.108.533315
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28(4):399–414. doi:10.1179/016164106x115008
Matz PG, Fujimura M, Chan PH (2000) Subarachnoid hemolysate produces DNA fragmentation in a pattern similar to apoptosis in mouse brain. Brain Res 858(2):312–319
He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH (2012) CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke 43(2):484–490. doi:10.1161/strokeaha.111.626432
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276(11):7806–7810. doi:10.1074/jbc.M008922200
Oliveira SJ, de Sousa M, Pinto JP (2011) ER stress and iron homeostasis: a new frontier for the UPR. Biochem Res Int 2011:896474. doi:10.1155/2011/896474
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306(5704):2090–2093. doi:10.1126/science.1104742
Li L, Holscher C, Chen BB, Zhang ZF, Liu YZ (2011) Hepcidin treatment modulates the expression of divalent metal transporter-1, ceruloplasmin, and ferroportin-1 in the rat cerebral cortex and hippocampus. Biol Trace Elem Res 143(3):1581–1593. doi:10.1007/s12011-011-8967-3
Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O (2001) A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276(11):7811–7819. doi:10.1074/jbc.M008923200
Frazer DM, Anderson GJ (2003) The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis 30(3):288–297
Qian ZM, Wang Q (1998) Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Res Brain Res Rev 27(3):257–267
Klomp LW, Farhangrazi ZS, Dugan LL, Gitlin JD (1996) Ceruloplasmin gene expression in the murine central nervous system. J Clin Invest 98(1):207–215. doi:10.1172/jci118768
Wang Z, Meng CJ, Shen XM et al (2012) Potential contribution of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 to blood-brain barrier disruption and brain edema after experimental subarachnoid hemorrhage. J Mol Neurosci 48(1):273–280. doi:10.1007/s12031-012-9769-6
Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G (2011) Role of iron in brain injury after intraventricular hemorrhage. Stroke 42(2):465–470. doi:10.1161/strokeaha.110.602755
Hockel K, Trabold R, Scholler K, Torok E, Plesnila N (2012) Impact of anesthesia on pathophysiology and mortality following subarachnoid hemorrhage in rats. Exp Transl Stroke Med 4(1):5. doi:10.1186/2040-7378-4-5
Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K, Zhang JH (2006) Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke 37(7):1888–1894. doi:10.1161/01.str.0000227259.15506.24
Desland FA, Afzal A, Warraich Z, Mocco J (2014) Manual versus automated rodent behavioral assessment: comparing efficacy and ease of bederson and garcia neurological deficit scores to an open field video-tracking system. J Cent Nerv Syst Dis 6:7–14. doi:10.4137/JCNSD.S13194
Wang SM, Fu LJ, Duan XL, Crooks DR, Yu P, Qian ZM, Di XJ, Li J, Rouault TA, Chang YZ (2010) Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci 67(1):123–133. doi:10.1007/s00018-009-0167-3
Horky LL, Pluta RM, Boock RJ, Oldfield EH (1998) Role of ferrous iron chelator 2,2′-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg 88(2):298–303. doi:10.3171/jns.1998.88.2.0298
Chi SI, Wang CK, Chen JJ, Chau LY, Lin TN (2000) Differential regulation of H- and L-ferritin messenger RNA subunits, ferritin protein and iron following focal cerebral ischemia-reperfusion. Neuroscience 100(3):475–484
Gitlin JD (1998) Aceruloplasminemia. Pediatr Res 44(3):271–276. doi:10.1203/00006450-199809000-00001
Jomova K, Vondrakova D, Lawson M, Valko M (2010) Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345(1–2):91–104. doi:10.1007/s11010-010-0563-x
Ong WY, Halliwell B (2004) Iron, atherosclerosis, and neurodegeneration: a key role for cholesterol in promoting iron-dependent oxidative damage? Ann N Y Acad Sci 1012:51–64
Ghribi O, Golovko MY, Larsen B, Schrag M, Murphy EJ (2006) Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem 99(2):438–449. doi:10.1111/j.1471-4159.2006.04079.x
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21(1):2–14. doi:10.1097/00004647-200101000-00002
Yeo JE, Kang SK (2007) Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta 1772(11–12):1199–1210. doi:10.1016/j.bbadis.2007.09.004
Nguyen TT, Cho SO, Ban JY, Kim JY, Ju HS, Koh SB, Song KS, Seong YH (2008) Neuroprotective effect of Sanguisorbae radix against oxidative stress-induced brain damage: in vitro and in vivo. Biol Pharm Bull 31(11):2028–2035
Matz PG, Fujimura M, Lewen A, Morita-Fujimura Y, Chan PH (2001) Increased cytochrome c-mediated DNA fragmentation and cell death in manganese-superoxide dismutase-deficient mice after exposure to subarachnoid hemolysate. Stroke 32(2):506–515
He Z, Ostrowski RP, Sun X, Ma Q, Tang J, Zhang JH (2012) Targeting C/EBP homologous protein with siRNA attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Exp Neurol 238(2):218–224. doi:10.1016/j.expneurol.2012.08.025
Acknowledgments
This study was supported by the National Natural Science Foundation of China, National Clinical key specialty construction projects of China and funded project of Chongqing Science and Technology Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The animal experimental ethics committee of Chongqing Medical University approved the experimental protocol.
Rights and permissions
About this article
Cite this article
Tan, G., Liu, L., He, Z. et al. Role of hepcidin and its downstream proteins in early brain injury after experimental subarachnoid hemorrhage in rats. Mol Cell Biochem 418, 31–38 (2016). https://doi.org/10.1007/s11010-016-2730-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2730-1