Abstract
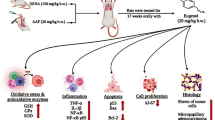
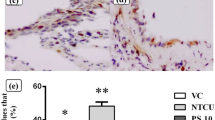
Roles of cyclooxygenase (COX) enzyme and intrinsic pathway of apoptosis have been explored for the chemopreventive effects of non-steroidal anti-inflammatory drugs (NSAIDs) on 9,10-dimethyl benz(a)anthracene (DMBA)-induced lung cancer in rat model. 16 weeks after the administration of DMBA, morphological analysis revealed the occurrences of tumours and lesions, which were regressed considerably with the co-administration of indomethacin and etoricoxib, the two NSAIDs under investigation. DMBA group was marked by hyperplasia and dysplasia as observed by histological examination, and these features were corrected to a large extent by the two NSAIDs. Elevated levels of COX-2 were seen in the DMBA group, the enzyme responsible for prostaglandin synthesis during inflammation and cancer, whilst the expression of the constitutive isoform, COX-1, was equally expressed in all the groups. Apoptosis was quantified by studying the activities of apaf-1, caspase-9, and 3 by immunofluorescence and western blots. Their activities were found to diminish in the DMBA-treated animals as compared to the other groups. Fluorescent co-staining of the isolated broncho-alveolar lavage cells showed reduced number of apoptotic cells in the DMBA group, indicating decrease in apoptosis after carcinogen administration. The present results thus suggest that the mechanism of cancer chemoprevention of NSAIDs may include the suppression of COX-2 and the induction of apoptosis.







Similar content being viewed by others
References
Hirsch FR, Lipmann SM (2005) Advances in the biology of lung cancer chemoprevention. J Clin Oncol 23:3186–3197
Soria JC, Kim ES, Fayette J, Lantuejoul S, Deutcsh E, Hong WK (2003) Chemoprevention of lung cancer. Lancet Oncol 4:659–669
Lubin JH, Caporaso NE (2006) Cigarette smoking and lung cancer: modelling total exposure and intensity. Cancer Epidemiol Biomarkers Prev 15:517–523
Zhong L, Goldberg MS, Parent ME, Hanley JA (2000) Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer 27:3–18
Zandwijk NV, Hirsch FR (2003) Chemoprevention of lung cancer: current status and future prospects. Lung Cancer 42:S71–S79
Li N, Chen X, Liao J, Yang G, Wang S, Josephson Y, Chi H, Chen J, Huang MT, Yang CS (2003) Inhibition of 7,12-dimethyl benz(a)anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23:1307–1313
Buters J, Martinez LQ, Schober W, Soballa VJ, Hintermair J, Wolff T, Gonzalez FJ, Greim H (2003) CYP1B1 determines susceptibility to low doses of 7,12-dimethyl benz(a)anthracene induced ovarian cancers in mice: correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis 24:327–334
Han BS, Fukamachi K, Takasuka N, Ohnishi T, Maeda M, Yamasaki T, Tsuda H (2002) Inhibitory effects of 17-estradiol and 4-n-octylphenol on 7,12-dimethyl benz(a)anthracene induced mammary tumor development in human c–Ha-ras protooncogene transgenic rats. Carcinogenesis 23:1209–1215
Walters MA, Roe FJC, Levene A (1967) The induction of tumours and other lesions in hamsters by a single sub-cutaneous injection of 9,10-dimethyl-l,2-benzanthracene or urethane on the first day of life. Br J Cancer 21:184–189
Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K (1999) Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res 59:198–204
Chan TA, Morin PJ, Vogelstein B, Kinzler KW (1998) Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA 95:681–686
Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristimäki A (1998) Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 58:4997–5001
Rishikesh MK, Sadhana SS (2003) Prostaglandins and cyclooxygenase: their probable roles in cancer. Indian J Pharmacol 35:3–12
Petkova DK, Clelland C, Ronan J, Pang L, Coulson JM, Lewis S, Knox AJ (2004) Over-expression of cyclooxygenase-2 in non-small cell lung cancer. Respir Med 98:164–172
Huang C, Taki T, Adachi M, Konishi T, Higashiyama M, Miyake M (1998) Mutations in exon 7 and 8 of p53 as poor prognostic factors in patients with non-small cell lung cancer. Oncogene 16:2469–2477
Hofer M, Hoferová Z, Fedorocko P, Macková NO (2002) Hematopoiesis-stimulating and anti-tumor effects of repeated administration of diclofenac in mice with transplanted fibrosarcoma cells. Physiol Res 51:629–632
Lee S, Krisanapun C, Baek SJ (2010) NSAID activated gene-1 as a molecular target for capsaicin induced apoptosis through a novel molecular mechanism involving GSK3β, C/EBPβ and ATF3. Carcinogenesis 31:719–728
Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25:4798–4811
Léveillé F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, Tolkovsky AM, Hardingham GE (2010) Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci 30:2623–2635
Saini RK, Sanyal SN (2008) Pulmonary carcinogenesis in mice with a single intratracheal instillation of 9,10-dimethyl benz(a)anthracene. Drug Chem Toxicol 31:459–471
Sodini D, Baragatti B, Barogi S, Laubach VE, Coceani F (2008) Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br J Pharmacol 153:1631–1640
Riendeau D, Percival MD, Brideau C, Charleson S, Dubé D, Ethier D, Falguryret JP, Friesen RW, Gordon R, Greig G, Guay J, Mancini J, Ouellet M, Wong E, Xu L, Boyce S, Visco D, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson AW, Young RN, Chan CC (2001) Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther 296:558–566
Pearse AGE (1968) Histochemistry, theoretical and applied, vol 1, 3rd edn. Churchill Livingstone, London, p 660
Sambrook J, Fritsch EF, Maniatis T (1998) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York, p 192
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J (1994) Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest 94:2105–2116
Yuan YJ, Ge ZQ, Li JC, Wu JC, Hu ZD (2002) Differentiation of apoptotic and necrotic cells in suspension cultures of Taxus cuspidata by the combined use of fluorescent dying and histochemical staining methods. Biotechnol Lett 24:7–76
Lehrach H, Diamond D, Wozney JM, Boedtker H (1977) mRNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry 16:4743–4751
Campa D, Zienolddiny S, Maggini V, Skaug V, Haugen A, Canzian F (2004) Association of a common polymorphism in the cyclooxygenase 2 gene with risk of non-small cell lung cancer. Carcinogenesis 25:229–235
Shaik MS, Chatterjee A, Singh M (2004) Effect of a selective cyclooxygenase-2 inhibitor, nimesulide, on the growth of lung tumors and their expression of Cyclooxygenase-2 and peroxisome proliferator- activated receptor-gamma. Clin Cancer Res 10:1521–1529
Harris RE, Beebe-Donk J, Schuller HM (2002) Chemoprevention of lung cancer by non-steroidal anti-inflammatory drugs among cigarette smokers. Oncol Rep 9:693–705
Shishodia S, Aggarwal BB (2004) Nuclear factor-kappaB: a friend or a foe in cancer? Biochem Pharmacol 68:1071–1080
Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Wang N, Spinka CM, Carroll RJ, Turner ND, Chapkin RS, Lupton JR (2004) An increase in reactive oxygen species by dietary fish oil coupled with the attenuation of antioxidant defenses by dietary pectin enhances rat colonocyte apoptosis. J Nutr 134:3233–3238
Haynes A, Shaik MS, Chatterjee A, Singh M (2003) Evaluation of an aerosolized selective COX-2 inhibitor as a potentiator of doxorubicin in a non-small-cell lung cancer cell line. Pharm Res 20:1485–1495
Keller JJ, Giardiello FM (2003) Chemoprevention strategies using NSAIDs and COX-2 inhibitors. Cancer Biol Ther 2:S140–S149
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Call JA, Eckhardt SG, Camidge DR (2008) Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol 9:1002–1011
Schick AR, Shoeneck A, Graeven U et al (2003) Mesalazine causes a mitotic arrest and induces caspase dependent apoptosis in colon carcinoma cells. Carcinogenesis 24:443–451
Kaur J, Sanyal SN (2010) PI3-kinase/Wnt association mediates COX-2/PGE2 pathway to inhibit apoptosis in early stages of colon carcinogenesis: chemoprevention by diclofenac. Tumor Biol 31:623–631
Vaish V, Tanwar L, Kaur J, Sanyal SN (2010) Chemopreventive effects of non-steroidal anti-inflammatory drugs in early neoplasm of experimental colorectal cancer: An apoptosome study. J Gastrointest Cancer 42:195–203
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196:254–265
Montuenga LM, Pio R (2007) Tumour-associated macrophages in nonsmall cell lung cancer: the role of interleukin-10. Eur Respir J 30:608–610
Higuchi Y (2004) Glutathione depletion-induced chromosomal DNA fragmentation associated with apoptosis and necrosis. J Cell Mol Med 8:455–464
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Setia, S., Vaish, V. & Sanyal, S.N. Chemopreventive effects of NSAIDs as inhibitors of cyclooxygenase-2 and inducers of apoptosis in experimental lung carcinogenesis. Mol Cell Biochem 366, 89–99 (2012). https://doi.org/10.1007/s11010-012-1286-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1286-y