Abstract
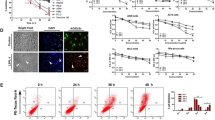
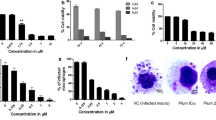
Lipids, especially sphingolipids, are emerging as inducer of apoptosis in a wide range of immortal cells, potentiating their therapeutic application in cancer. In the present study, a sphingolipid rich lipid fraction (denoted here as ALL), isolated from an attenuated strain of Leishmania donovani promastigote, was tested for its tumoricidal activity taking melanoma, the dreaded form of skin cancer cells, as model. ALL was found to induce chromatin condensation, internucleosomal DNA fragmentation and phosphatidylserine externalization with enhanced cell population in sub-G1 region in both mouse and human melanoma systems, namely B16F10 and A375 respectively. These are the hallmarks of cells undergoing apoptosis. Further analysis demonstrated that ALL treated melanoma cells showed significant increase in ROS generation, mitochondrial membrane potential depolarization, release of cytochrome c, and caspase-3 activation, which are the events closely involved in apoptosis. These findings indicate that one or more bioactive sphingolipid(s)/ceramide(s) present in ALL could be the causative agent(s) for the induction of apoptosis in melanoma cells. Further studies are thus necessary to identify these specific bioactive sphingolipid(s)/ceramide(s) and to establish their mechanism of action, in order to explore their use as anticancer agents.
Similar content being viewed by others
References
Spiegel S, Milstien S: Sphingolipid metabolites: members of a new class of lipid second messengers. J Membr Biol 146: 225–237, 1995
Mandala SM, Thornton R, Tu Z, Kurdz MB, Nickels J, Broach J, Menzaleev R, Spiegel S: Sphingolipid base-1-phosphate phophatase: A key regulator of sphingolipid metabolism and stress response. Proc Natl Acad Sci USA 95: 150–155, 1998
Kerr JF, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer 26: 239–257, 1972
Marx J: Cell death studies yield cancer clues Science. (Washington DC) 259: 760–761, 1993
Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462, 1995
Giorgio S, Santos MRM, Straus AH, Takahasi HK, Barbieri CL: Effect of Glycosphingolipids purified from Leishmania (Leishmania) amazonensis Amastigotes on Human Peripheral Lymphocytes. Clin Diagn Lab Immunol 3: 469–472, 2003
Banerjee A, Saha KD, Bera R, Ratha J, Basu K, Bhadra R: Sphingolipids from Leishmania donovani modulates responses of synovial fluid cells of rheumatoid arthritis patients. 10th FAOBMB Congress Poster Abstracts, Bangalore, India, December 7–11, 2003.
Douglas G, Dario CA: Drug resistance in melanoma: Mechanisms, apoptosis, and new potential therapeutic targets. Cancer and Metastasis Rev 20: 3–11, 2001
Ray JC: Cultivation of various Leishmania parasites on solid medium. Ind J Med Res 20: 355–357, 1932
Handman E: Leishmania vaccines: Old and new. Parasitol Today 13: 236–238, 1997
Mukhopadhyaya S, Bhattacharya S, Majhi R, De T, Naskar K, Majumdar S, Roy S: Use of an Attenuated Leishmanial Parasite as an Immunoprophylactic and Immunotherapeutic Agent against Murine Visceral Leishmaniasis. Clin Diagn Lab Immunol 7: 233–240, 2000
Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959
Dasgupta S, Hogan EL: Chromatographic resolution and quantitative assay of CNS tissue sphingoids and sphingolipids. J Lipid Res 42: 301–308, 2001
Bischel MD, Austin JH: A modified benzidine method for the chromatographic detection of sphingolipids and acid polysaccharides. Biochim Biophys Acta 70: 598–600, 1963
Zhang H, Desai NN, Murphey JM, Spiegel S: Sphingosine stimulates cellular proliferation via a protein kinase C-independent pathway. J Biol Chem 265: 21309–21316, 1990
Mossman T: Rapid colorimetric assay for cellular growth and survival. J Immunol Method 65: 55, 1983
Hongo T, Mizuno Y, Haraguchi S, Yoshida TO: A new anticancer drugs sensitivity test using the microplate culture and surviving tumor cell staining methods. Gan To Kagaku Ryoho 13: 247–254, 1986
Cohen JJ: Apoptosis. Immunol Today 14: 126–130, 1993
Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Geltinger C, Nakahara T, Takehide M, Yokoyama KK: Mannosylerythritol Lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res 59: 482–486,1999
Huang X, Frenkel K, Klein C, Costa M: Nickel induced oxidants in intact cultured mammalian cells as detected by dichlorofluorescein fluorescence. Pharmacol Toxicol 120: 29–36, 1993
Bhosle SM, Huilgol NG, Mishra KP: Enhancement of radiation induced oxidative stress and cytotoxicity in tumor cells by ellagic acid. Clinica Chimia Acta 359: 89–100, 2005
Vermes I, Haanen C, Stiffens-Nakken H, Reutellingsperger C: Flow cytometric detection of phosphatidyl serine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods 184: 39–51, 1995
Buttke TM, Sandstrom PA: Oxidative stress as a mediator of apoptosis. Immunol Today 15: 7–10, 1994
Aruoma OI, Halliwell B, Hoey BM, Butler J: The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free Radical Biol Med 6: 593–597, 1989
Searle J, Kerr JFR, Bishop CJ: Necrosis and Apoptosis: Distinct mode of cell death with fundamentally different significance. Pathol Ann 17: 229–259, 1982
Benson RS, Heer S, Drive C, Watson AJ: Characterization of cell volume loss in CEM-C7A cells during dexamethason induced apoptosis. Am J Physol 270: C1190–C1203, 1996
Allen RT, Hunter III WJ, Agrawal DK: Morphological and Biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods 37: 215–228, 1997
Eamshaw WC: Nuclear changes in apoptosis. Curr Opin Cell Biol 7: 337–343, 1995
Gracia RC, Colell A, Mari M, Morales A, Fernadez-Checa J: Direct interaction of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. J Biol Chem 272: 11369–11377, 1997
Quillet-Mary A, Jaffrezou JP, Mansal V, Bordier C, Naval J, Laurent G: Implication of mitochondrial hydrogen peroxide generation in ceramide induced apoptosis. J Biol Chem 272: 21388–21395, 1997
Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ: Ceramide induces hepatocytes cell death through disruption of mitochondrial function in the rat. Hepatology 25: 957–963, 1997
Crompton M: The mitochondrial permeability transition pore and its role in cell death. Biochem J 341: 233–249, 1999
Cai J, Yang J, Jones DP: Mitochondrial control of apoptosis: The role of cytochrome c. Biochem Biophys Acta 1366: 139–149, 1998
Cohen GM: Caspases: the executioners of apoptosis. Biochem J 326: 1–16, 1997
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X: Cytochrome c and dATP dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489, 1997
Hung WC, Chang HC, Chuang LY: Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem J 338: 161–166, 1999
Endo K, Igarashi Y, Nisar M, Zhou Q, Hakomori S: Cell membrane signaling as target in cancer therapy: inhibitory effect of N,N-dimethyl and N,N,N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res 51: 1613–1618, 1991
Sadahira Y, Ruan F, Hakomori S, Igarashi Y: Sphingosine1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci USA 89: 9686–9690, 1992
Shirahama T, Sweeny EA, Sahakura C, Singhal AK, Nishiyama K, Akiyama S, Hakomori S, Igarashi Y: In vitro and in vivo induction of apoptosis by sphingosine and N, N-dimethylsphingosine in human epidermoid carcinoma KB-3-1 and its multidrug-resistant cells. Clin Cancer Res 3: 257–264, 1997
Sweeny EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, Igarashi Y: Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer 66: 358–366, 1996
Raisova M, Bektas M, Wieder T, Daniel P, Eberle J, Orfanos CE, Geilen CC: Resistance to CD95/Fas-induced and ceramide mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. FEBS Letters 473: 27–32, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratha, J., Majumdar, K.N., Mandal, S.K. et al. A sphingolipid rich lipid fraction isolated from attenuated Leishmania donovani promastigote induces apoptosis in mouse and human melanoma cells in vitro . Mol Cell Biochem 290, 113–123 (2006). https://doi.org/10.1007/s11010-006-9174-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9174-y