Abstract
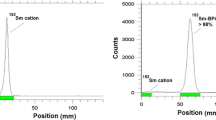
Developing new bone pain palliation agents are a mandate in handling end-stage cancer patient’s around the world. 177Lu (1-hydroxy-2-imidazol-1-yl-phosphonoethyl)phosphonic acid (177Lu–ZLD) is a possible therapeutic agent which can be used in bone palliation therapy. In this study, 177Lu–ZLD complex was prepared successfully using commercial ZLD ligand and 177LuCl3 at 25 and 60 °C at various ligand:metal ratios for 60–360 min. 177Lu chloride was obtained by thermal neutron irradiation (4 × 1013 n cm−2s−1) of natural Lu2O3 samples. Radiochemical purity of 177Lu–ZLD was checked by ITLC and HPLC. Stability studies of final preparation in the presence of human serum were performed as well as protein binding studies and hydroxyapatite (HA) binding test. The biodistribution of 177Lu–ZLD and 177LuCl3 in mice were determined for 7 days. A comparative accumulation study for 177Lu–ZLD and 177Lu–EDTMP was performed for vital organs up to 7 days. The complex was obtained in high radiochemical purity ITLC (>97 %) and HPLC (>99.9 %) and satisfactory stability in presence of human serum and final formulations were obtained (≈90 % in 48 h). HA binding assay demonstrated >95 % binding from 5 to 20 mg of HA in 24 h at room temperature. The complex protein binding was about 55–58 %. The high bone uptake ratios at all time intervals was obtained (>9 % at day 7), bone:kidney and bone:liver uptake ratios were significantly high for ZLD at 7 day post injection but not superior to 177Lu–EDTMP. Due to longer physical half life of 177Lu compared to 153Sm and comparable ratios for 177Lu–ZLD compared to 177Lu–EDTMP, 177Lu–ZLD can be an interesting new candidate for clinical trials for bone pain palliation therapy.











Similar content being viewed by others
References
Serafini AN (2001) Therapy of metastatic bone pain. J Nucl Med 42:895–906
Bayouth JE, Macey DJ, Kasi LP, Fossella FV (1994) Dosimetry and toxicity of Samarium-153–EDTMP administered for bone pain to skeletal metastases. J Nucl Med 35:63–69
Campa JA, Rayne R (1992) The management of intractable bone pain: a clinician’s perspective. Semin Nucl Med 22:3–10
Eary JF, Collin C, Stabin M, Vernon C, Petersdorf S, Baker M, Hartnett S, Ferency S, Addison SJ, Appelbaum F (1993) Samarium-153–EDTMP biodistribution and dosimetry estimation. J Nucl Med 34:1031–1036
Luegmayr C-TE, Freedman LP, Guideon A, Rodan L (2006) Relative binding affinities of bisphosphonates for human bone and relationship to antiresorptive efficacy. Bone 38(5):628–636
http://www.theodora.com/drugs/zometa_for_intravenous_infusion_novartis.html
Lin J, Qiu L, Cheng W, Luo S, Xue L, Zhang S (2012) Development of superior bone scintigraphic agent from a series of 99mTc-labeled zoledronic acid derivatives. Appl Radiat Isot 70(5):848–855
Majkowska A, Neves M, Ines Antunes I, Aleksander Bilewicz A (2009) Complexes of low energy beta emitters 47Sc and 177Lu with zoledronic acid for bone pain therapy. Appl Radiat Isot 67:11–13
McKenzie K, Eng M, Bobyn JD, Roberts J, Karabasz D, Tanzer M (2011) Bisphosphonate remains highly localized after elution from porous implants. Clin Orthop Relat Res 469:514–522
Chakraborty S, Das T, Banerjee S, Balogh L, Chaudhari PR, Sarma HD, Polyák A, Máthé D, Venkatesh M, Janoki G, Pillai MR (2008) 177Lu–EDTMP: a viable bone pain palliative in skeletal metastasis. Cancer Biother Radiopharm 23:202–213
Manual for reactor produced radioisotopes, International Atomic Energy Agency (IAEA), Vienna, 2003, IAEA-TECDOC-1340, ISBN 92–0–101103–2, ISSN 1011–4289, pp 121–123, Printed by the IAEA in Austria, January 2003
cGRPP-guidelines (2007) EANM Radiopharmacy Committee, guidelines on current good radiopharmacy, practice (CGRPP) in the preparation of radiopharmaceuticals, version 2 March 2007
Dar UK, Khan IU, Javed M, Ahmad F, Ali M, Hyder SW (2012) Preparation and biodistribution in mice of a new radiopharmaceutical technetium-99m labeled methotrexate, as a tumor diagnostic agent. Hell J Nucl Med 15(2):120–124
Neves M, Gano L, Pereira N, Costa MC, Costa MR, Chandia M, Rosado M, Fausto R (2002) Synthesis, characterization and biodistribution of bisphosphonates Sm-153 complexes: correlation with molecular modeling interaction studies. Nucl Med Biol 29:329–338
Du XL, Zhang TL, Yuan L, Zhao YY, Li RC, Wang K, Yan SC, Zhang L, Sun H, Qian ZM (2002) Complexation of ytterbium to human transferrin and its uptake by K562 cells. Eur J Biochem 269:6082–6090
Yousefnia H, Anvari A, Bahrami-Samani A, Jalilian AR, Shirvani-Arani S, Aghamiri MR, Ghannadi Maragheh M (2012) Production, quality control and pharmacokinetic studies of 177Lu–EDTMP for human bone pain palliation therapy trials. Iran J Pharmaceut Res 11(1):137–144
Yuan J, Liu C, Liu X, Wang Y, Kuai D, Zhang G, Zaknun JJ (2013) Efficacy and safety of 177Lu–EDTMP in bone metastatic pain palliation in breast cancer and hormone refractory prostate cancer: a phase II study. Clin Nucl Med 38(2):88–92
Acknowledgments
We acknowledge the financial support of Iran National Science Foundation (INSF) for conducting this research project. The authors wish to thank Mr. M. Mazidi for performing animal tests as well as Dr. M. Erfani for HPLC experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikzad, M., Jalilian, A.R., Shirvani-Arani, S. et al. Production, quality control and pharmacokinetic studies of 177Lu–zoledronate for bone pain palliation therapy. J Radioanal Nucl Chem 298, 1273–1281 (2013). https://doi.org/10.1007/s10967-013-2490-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2490-2