Abstract
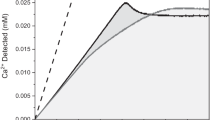
The metastable and stable equilibria during the precipitation of calcium phosphates in three biomimetic systems, namely SBFc-CaCl2–K2HPO4–H2O, SBFc-CaCl2–MgCl2–K2HPO4–H2O and SBFc-CaCl2–ZnCl2–K2HPO4–H2O, where SBFc denotes a conventional simulated body fluid, were modeled by a thermodynamic approach (ion-association model, computer program PHREEQCI v.2.14.3). In all cases the highest saturation indices (SI) and the thermodynamic stability were calculated for hydroxyapatite, Ca5(PO4)3OH. Co-precipitation of metastable phases of seven salts in the first system, co-precipitation of additional four magnesium salts in the second system, and no co-precipitation of zinc salts in the third system were calculated at pH 8. Precipitation of amorphous calcium phosphate incorporating Mg2+, Na+, K+, \( {\text{CO}}_{3}^{2 - } \) and Cl− ions at levels close to those of natural enamel, dentin and bone, instead of the thermodynamically stable hydroxyapatite, was experimentally found. This reveals that kinetic factors are decisive for the precipitation processes. In addition, the phase transformations of the precipitated metastable amorphous products (9.94 > SI > 0) during their maturation in the three simulated body fluids, differing in their concentrations of \( {\text{HCO}}_{3}^{ - } \) and Cl− ions and organic macromolecules, were also modeled. Dissolution of all magnesium salts was found to occur and equilibrium of the more stable calcium salts was established, depending on the \( {\text{HCO}}_{3}^{ - } \) concentration. In contrast, transformation of the stable equilibrium products (SI > 0) to the thermodynamically stable hydroxyapatite (SI = 0), independent of the \( {\text{HCO}}_{3}^{ - } \) concentration, was both calculated and experimentally proven. Analogous distribution of the species was calculated for the initial and equilibrium solutions of the studied SBFs, while it was different for the metastable solutions. The predominance of free Me2+ ions (Me = Ca, Mg) was calculated in all studied cases.






Similar content being viewed by others
References
Pana, H., Zhaoa, X., Brian Darvell, W., Lua, W.W.: Apatite-formation ability—predictor of “bioactivity”. Acta Biomater. 6, 4181–4188 (2010)
Al-Haddad, A., Kutty, M.G., Abu Kasim, N.H., Ab Aziz, Z.A.C.: Physicochemical properties of calcium phosphate based coating on gutta-percha root canal filling. Int. J. Polym. Sci. (2015). doi:10.1155/2015/414521
Oyane, A.: Development of apatite-based composites by a biomimetic process for biomedical applications. J. Ceram. Soc. Jpn. 118, 77–81 (2010)
Jalota, S., Bhaduri, S.B., Tas, A.C.: Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions. J. Mater. Sci.: Mater. Med. 17, 697–707 (2006)
Earle, W.R., Schilling, E.L., Stark, T.H., Straus, N.P., Brown, M.F., Shelton, E.: Production of malignancy in vitro. IV. The mouse fibroblast cultures and changes seen in the living cells. J. Natl. Cancer Inst. 4, 165–212 (1943)
Hanks, J.H., Wallace, R.E.: Relation of oxygen and temperature in the preservation of tissues by refrigeration. Proc. Soc. Exp. Biol. Med. 71, 196–200 (1949)
Kokubo, T.: Surface chemistry of bioactive glass-ceramics. J. Non-Crystalline Solids 120, 138–151 (1990)
Bayraktar, D., Tas, A.C.: Chemical preparation of carbonated calcium hydroxyapatite powders at 37 °C in urea-containing synthetic body fluids. J. Eur. Ceram. Soc. 19, 2573–2579 (1999)
Marques, P.A.A.P., Serro, A.P., Saramago, B.J., Fernandes, A.C., Magalhaes, M.C.F., Correia, R.N.: Mineralisation of two calcium phosphate ceramics in biological model fluids. J. Mater. Chem. 13, 1484–1490 (2003)
Oyane, A., Kim, H.M., Furuya, T., Kokubo, T., Miyazaki, T., Nakamura, T.: Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res., Part A 65, 188–195 (2003)
Kim, H.M., Miyazaki, T., Kokubo, T., Nakamura, T.: Revised simulated body fluid. Key Eng. Mater. 192, 47–50 (2000)
Glinkina, I.V., Durov, V.A., Mel’nitchenko, G.A.: Modelling of electrolyte mixtures with application to chemical equilibria in mixtures—prototypes of blood’s plasma and calcification of soft tissues. J. Mol. Liq. 110, 63–67 (2004)
Pasteris, J.D., Wopenka, B., Valsami-Jones, E.: Bone and tooth mineralization: why apatite. Elements 4, 97–104 (2008)
Lu, X., Leng, Y.: Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 26, 1097–1108 (2005)
Chow, L.C., Eanes, E.D.: Octacalcium Phosphate. Karger, Basel (2001)
Johnsson, M.S.-A., Nancollas, G.H.: The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 3, 61–82 (1992)
Parkhurst, D.L.: User’s guide to PHREEQC—a computer program for speciation, reaction-path, advective-transport, and inverse geochemical calculations. U.S. Geological Survey Water-Resources Investigations Report. 95-4227 (1995). http://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqci/
Ball, J.W., Nordstrom, D.K.: WATEQ4F-User’s manual, U.S. Geological Survey Open-File Report 90-129 (1991)
Allison, J.D., Brown D.S., Novo-Gradac K.J.: MINTEQA2/PRODEFA2—User’s Manual. Environmental Research Laboratory, Office of Research and Development, U.S. EPA, Athens (1990)
Charlton S.R., Parkhurst, D.L.: PHREEQCI-A graphical user interface to the geochemical model PHREEQC: U.S. Geological Survey Fact Sheet. FS-031-02 (2002)
Gustafsson J.P.: Visual MINTEQ ver. 3.0 (2010). http://www2.lwr.kth.se/English/OurSoftware/vminteq/index.htm
Magalhaes, M.C., de Aguiar Pereira Marques, P.A., Correia, R.: Calcium and magnesium phosphates: normal and pathological mineralization. In: Biomineralization—Medical Aspects of Solubility, pp. 71–123. Wiley, New York (2006)
Hamad, M., Heughebaert, J.C.: The growth of whitlockite. J. Cryst. Growth 79, 192–197 (1986)
McDowell, H., Gregory, T.M., Brown, W.E.: Solubility of Ca5(PO4)3OH in the system Ca(OH)2–H3PO4–H2O at 5, 15, 25 and 37 °C. J. Res. Natl. Bur. Stand. Sect. A 81A, 273–281 (1977)
Sykora, V.: Chemicko Analyticke Tabulky, p. 157, Praha (1976)
Boanini, E., Gazzano, M., Bigi, A.: Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 6, 1882–1894 (2010)
Yamaguchi, M.: Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. 11, 119–135 (1998)
Moonga, B.S., Dempster, D.W.: Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J. Bone Miner Res. 10, 453–457 (1995)
Dorozhkin, S.V.: Calcium orthophosphates in nature, biology and medicine. Materials 2, 399–498 (2009)
Betts, F., Posner, A.F.: Structural model for amorphous calcium phosphate. Trans. Am. Crystallogr. Assoc. 10, 73–84 (1974)
Treboux, G., Layrolle, P., Kanzaki, N., Onuma, K., Ito, A.: Existence of Posner’s cluster in vacuum. J. Phys. Chem. A 104, 5111–5114 (2000)
Christoffersen, M.R., Christoffersen, J., Kibalczyc, W.: Apparent solubilities of two amorphous calcium phosphates and of octacalcium phosphate in the temperature range 30–42 °C. J. Cryst. Growth 106, 349–354 (1990)
Dorozhkin, S.V.: Amorphous calcium (ortho)phosphates. Acta Biomater. 6, 4457–4475 (2010)
Acknowledgements
This work was financially supported by the Bulgarian Ministry of Education and Science under Project DFNI T02-5/2014.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rabadjieva, D., Tepavitcharova, S., Sezanova, K. et al. Chemical Equilibria Modeling of Calcium Phosphate Precipitation and Transformation in Simulated Physiological Solutions. J Solution Chem 45, 1620–1633 (2016). https://doi.org/10.1007/s10953-016-0528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0528-0