Abstract
Purpose
Standard therapy for lupus nephritis is based on non-specific immunosuppression. We aimed to identify specific alterations in T cell and cytokine homeostasis and possible associations with disease activity in children with lupus nephritis (LN).
Methods
The phenotype of circulating T cells from children with LN and healthy controls (HC) was analyzed by flow cytometry. Intracellular expression of IL-17 and INF-γ was assessed after stimulation with anti-CD3 and anti-CD28. Serum concentrations of IP10, CCL2, TGF-β, IL-17, and IL-23 were measured by ELISA. Disease activity was determined using the Systemic Lupus Erythematosus Disease Activity Index 2000 update (SLEDAI-2K).
Results
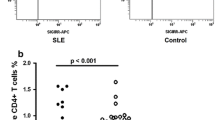
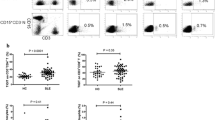
Children with active LN displayed increased frequencies of effector memory CD4+CD45RO+CCR7− and terminal differentiated CD4+CD45RA+CCR7− T cells and reduced naive CD4+CD45RA+CCR7+ T cells compared to those with inactive LN or HC. Circulating CD4+CXCR3+ and CD4+CCR2+ T cells correlated inversely with the renal SLEDAI-2K, whereas IP10 and CCL2 showed a positive correlation. Reduced CD4+Foxp3+ T cells and serum TFG-β levels in active LN were associated with high serum IL-17 and IL-23 levels and correlated inversely with the renal SLEDAI-2K (r = −0.5855, p = 0.0013 and r = −0.6246, p = 0.0005, respectively), whereas IL-17 and IL-23 correlated positively (r = 0.5516, p = 0.0029 and r = 0.6116, p = 0.0007, respectively). Expansion of Th17 and Th1/Th17 cells in children with LN was significantly greater than in HC (p = 0.0304 and p = 0.0067, respectively).
Conclusion
Children with active LN display high levels of pro-inflammatory cytokines associated with an increase in effector T cells and reduction of regulatory T cells. Therapeutic regulation of the aberrant cytokine profile might specifically interrupt pathogenic mechanisms.




Similar content being viewed by others
References
Crispin JC, Tsokos GC. Novel molecular targets in the treatment of systemic lupus erythematosus. Autoimmun Rev. 2008;7(3):256–61. doi:10.1016/j.autrev.2007.11.020.
Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37(1):100–9.
Crispin JC, Kyttaris VC, Juang YT, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29(3):110–5.
Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–6.
Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8− T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990;87(18):7020–4.
Dolff S, Quandt D, Wilde B, Feldkamp T, Hua F, Cai X, et al. Increased expression of costimulatory markers CD134 and CD80 on interleukin-17 producing T cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 12(4):R150. doi:10.1186/ar3100.
Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183(7):4693–704. doi:10.4049/jimmunol.0802626.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi:10.1016/j.cell.2008.05.009.
Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. 107(1):204–9. doi:10.1073/pnas.0903158107.
La Cava A. The busy life of regulatory T cells in systemic lupus erythematosus. Discov Med. 2009;8(40):13–7.
Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58(2):556–62.
Bartosh SM, Fine RN, Sullivan EK. Outcome after transplantation of young patients with systemic lupus erythematosus: a report of the North American pediatric renal transplant cooperative study. Transplantation. 2001;72(5):973–8.
Doria A, Vesco P, Zulian F, Gambari PF. The 1982 ARA/ACR criteria for the classification of systemic lupus erythematosus in pediatric and adult patients. Clin Exp Rheumatol. 1994;12(6):689–90.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi:10.1002/1529-0131(199709)40:9<1725::AID-ART29>3.0.CO;2-Y.
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–50.
Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–60. doi:10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4.
Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–91. doi:10.1111/j.1600-065X.2009.00766.x.
Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–10. doi:10.1182/blood-2007-01-070748.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi:10.1038/44385.
Worbs T, Forster R. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol. 2007;28(6):274–80. doi:10.1016/j.it.2007.04.002.
Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, et al. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur J Immunol. 2007;37(3):613–22. doi:10.1002/eji.200636656.
Kulkarni O, Anders HJ. Chemokines in lupus nephritis. Front Biosci. 2008;13:3312–20.
El-Shehaby A, Darweesh H, El-Khatib M, Momtaz M, Marzouk S, El-Shaarawy N, et al. Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J Clin Immunol. 2011;31(5):848–56. doi:10.1007/s10875-011-9555-1.
Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 2009;20(12):2518–24. doi:10.1681/ASN.2009030337.
Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21(3):274–80. doi:10.1016/j.coi.2009.05.021.
Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171–81.
Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–9. doi:10.1038/ni.1610.
Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178(11):6725–9.
McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–24. doi:10.1038/ni.1698.
Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183(5):3160–9. doi:10.4049/jimmunol.0900385.
Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–63. doi:10.1038/ni.1993.
Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–21. doi:10.1111/j.1749-6632.2009.05133.x.
Acknowledgments
This work was supported by OeNB Jubiläumsfonds Grant (13334) and Medizinischer Forschungsfonds Tirol grant to ME.
Disclosures
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is dedicated in memory of Prof. Dr. Lothar Bernd Zimmerhackl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Renal SLEDAI-2K, total SLEDAI-2K, and extra-renal manifestations of SLE at the time of sampling in children with active LN (PDF 56 kb)
Online Resource 2
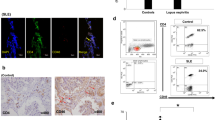
Representative FACS dot plots showing the frequencies of circulating CD45RA+CCR7− (A1), CD45RA+CCR7+ (A2), CD45RA−CCR7− (A3), and CD45RA−CCR7+ (A4) cells on gated CD4+ T cells from a patient with active lupus nephritis (16.9%, 29.4%, 34.5%, and 19.2%, respectively), inactive lupus nephritis (12.7%, 39.4%, 27.5%, and 20.4%, respectively), and a control subject (5.2%, 47.4%, 22.1%, and 25.3%, respectively) (PDF 158 kb)
Online Resource 3
Increased frequency of CD69 expressing DNT cells in childhood LN. Leukocytes were stained with monoclonal antibodies to CD16/56, CD3, CD4, CD8, and CD69. Representative FACS dot plots show b) the frequencies of CD3+CD4−CD8− T cells (DNT) on gated CD3+CD16/56− cells, numbers indicate the percentage of CD3+CD4−CD8− cells and c expression of CD69 on gated DNT cells, numbers indicate the percentage of double positive cells (PDF 1.30 mb)
Online Resource 4
Reduced circulating CD4+CXCR3+ T cells in active childhood-onset LN. Representative FACS dot plots of CXCR3 expression in gated CD4+ T cells from a child with active LN, inactive LN, nephrotic syndrome (NS), and a control subject. Numbers indicate the percentage of double positive cells (PDF 493 kb)
Online Resource 5
Decrease of circulating CD4+Foxp3+ T cells in active childhood-onset LN. Representative examples of Foxp3 expression in circulating CD4+ T cells of a patient with active LN, inactive LN, nephrotic syndrome (NS), and a control subject. Numbers indicate the percentage of double positive cells (PDF 245 kb)
Rights and permissions
About this article
Cite this article
Edelbauer, M., Kshirsagar, S., Riedl, M. et al. Activity of Childhood Lupus Nephritis is Linked to Altered T Cell and Cytokine Homeostasis. J Clin Immunol 32, 477–487 (2012). https://doi.org/10.1007/s10875-011-9637-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9637-0