Abstract
Two bacteriophages, ϕA38 and ϕA41, infecting Pectobacterium parmentieri strain SCC 3193 (former Pectobacterium wasabiae strain SCC 3193) were isolated from arable soil samples collected in different regions of Poland. ϕA38 and ϕA41 have a typical morphology of the members of the family Podoviride and order Caudovirales, with a head diameter of ca. 60 nm and tail length of ca. 20 nm. Phages ϕA38 and ϕA41 exhibited a similar RFLP pattern with Csp6I restriction endonuclease. They were stable in a range of pHs, temperatures and osmolarities but were rapidly inactivated by UV light. During the first 20 min., 74 and 69% of ϕA38 and ϕA41 phages, respectively, were adsorbed to SCC 3193 cells. In one-step growth experiments, ϕA38 and ϕA41 showed latent period of ca. 20–30 min and burst size of 102 and 141 phages, respectively. The optimal multiplicity of infection (MOI) was calculated to be 0.01 for both bacteriophages. In the host range experiments, both phages were able to infect six from 21 of the tested P. parmentieri isolates but the phages were unable to infect other members of the Pectobacterium spp. or Dickeya spp. In the proof-of-concept experiments, ϕA38 and ϕA41 were able to inhibit the growth of P. parmentieri strain SCC 3193 and to protect potato tuber tissue maceration caused by the bacterium. The potential use of ϕA38 and ϕA41 bacteriophages for the biocontrol of P. parmentieri in potato is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato blackleg and tuber soft rot caused by pectinolytic bacteria Pectobacterium and Dickeya species (also called soft rot Enterobacteriaceae – SRE) may result in important losses in (seed) potato production worldwide (Toth et al. 2011; Pérombelon 2002). To date, five SRE species are reported to cause potato blackleg in Europe viz. P. atrosepticum (Pba), D. dianthicola, D. solani, P. carotovorum subsp. brasiliense (Pcb) and P. parmentieri (former P. wasabiae) (Ppa, former Pwa) (de Haan et al. 2008; Toth et al. 2011; Waleron et al. 2013).
In 2010, P. wasabiae was described for the first time as a new pathogen of potato in New Zealand, responsible for high blackleg levels in field crops (Pitman et al. 2010). In the following years, P. wasabiae was isolated from symptomatic potato plants in different countries worldwide including Canada, New Zeeland, Iran, South Africa, Zimbabwe, Finland, France, Germany, Poland, the Netherlands, Serbia, Scotland and USA (Khayi et al. 2016). Likewise, a number of isolates classified previously as P. carotovorum were re-classified as P. wasabiae due to the advances in development of genome-based taxonomical methods. The results of these phylogenomic studies indicated that P. wasabiae was present in association with potato in Europe already for a long time and it is not an invasive Pectobacterium spp. recently introduced to Europe from outside (Khayi et al. 2016). Lately, it has been proposed to phylogenetically separate P. wasabiae strains isolated from potato and other hosts and collected in USA, Europe and Canada from strains infecting horseradish in Asia (Yuan et al. 2014; Pritchard et al. 2016). What is more, last year Khayi and co-workers (Khayi et al. 2016) reclassified potato-associated P. wasabiae strains to a new taxon named P. parmentieri (Khayi et al. 2016) - a distinct cluster from the one grouping P. wasabiae strains isolated from horseradish plants. In the last six years, potato-associated Pwa (now P. parmentieri) has continued to be an important causative agent of potato blackleg and soft rot in Europe leading to economically-important and increasing losses (van der Wolf et al. 2017).
Like in case of other soft rot Enterobacteriaceae, efficient control of Ppa with the use of traditional chemical and physical agents has not been achieved yet (Czajkowski et al. 2011). Similarly, there are no soft rot Enterobacteriaceae-immune potato cultivars available (Lapwood and Read 1986; Lapwood and Harris 1982) and it is doubtful whether such cultivars would appear on the market in the nearest future (Davidsson et al. 2013). Consequently, only hygienic practices, including but not limited to the use of certified seed material, avoidance of pathogen introduction in clean seed tubers and soil drainage to decrease oxygen depletion and water film on tubers planted in soil, can minimize the possibility of infection and spread of the pathogen in potato. These measures are, however, insufficient to eradicate blackleg and soft rot in potato entirely (van der Wolf and de Boer 2007; Czajkowski et al. 2011).
Bacteriophages (phages) are viruses able to infect and kill bacterial hosts (Duckworth and Gulig 2002). They are ubiquitous in all environments containing bacteria and have been found in soil, sewage, in and on animals and on plants (Abedon 2008; Gill and Abedon 2003). Bacteriophages have been proposed as potential biological control agents against plant pathogenic bacteria and consequently, they have been evaluated against different pathogens including Erwinia amylovora, Xanthomonas pruni, Pseudomonas tolaasii, Streptomyces scabies and Ralstonia solanacearum on different host plants (Jones et al. 2008, 2012). Similarly, phages were tested to control Pectobacterium spp. and Dickeya spp. but only limited attempts have been made to characterize these lytic bacteriophages in detail (Czajkowski et al. 2014, 2015; Adriaenssens et al. 2012). For example, no phages acting against Ppa have been reported in literature so far (Czajkowski 2016). The purpose of this study was to isolate and characterize novel lytic bacteriophages specific to P. parmentieri with the main idea to evaluate their interaction with bacterial host as well as phage potential as biological control agents.
Materials and methods
Bacterial strains and media
All bacterial strains used in this study are listed in Table 1. For routine tests and maintenance, bacteria were grown at 28 °C for 24–48 h on tryptone soya agar (TSA, Oxoid) prior to use. For liquid preparations, bacterial cultures were grown in tryptone soya broth (TSB, Oxoid) with agitation 200 rpm at 28 °C. For long-term preservation, bacterial cultures were kept in sterile 40% (v/v) glycerol at −80 °C.
Isolation of bacteriophages from environmental samples
Bulk soil and rhizosphere soil samples were collected between April and September 2013 in different arable regions in Poland. To isolate bacteriophages from the environmental samples we used protocol described previously (Czajkowski et al. 2014).
Enrichment of bacteriophages in their host bacteria and purification of individual phage particles
Pectobacterium parmentieri strain SCC 3193 (Pirhonen et al. 1988; Khayi et al. 2016) was used to enrich lytic bacteriophages from soil as previously described (Twest and Kropinski 2009). For this 1 ml of filter-sterilized soil extract was added to 9 ml of SCC 3193 bacterial culture grown in TSB, containing ca. 108 colony forming units (cfu) ml−1 and incubated at 28 °C for 24 h with shaking (200 rpm). After incubation, bacteria were removed by centrifugation (8000×g, 5 min) and supernatants were filter-sterilized with 0.22 μm filters (sterile active cellulose syringe filters, VWR). Purification of individual phage particles was done using a soft top agar method as previously described (Bertani 1951).
Bacteriophage morphology under transmission electron microscopy
Microscopic analyses were performed in the Laboratory of Electron Microscopy, Faculty of Biology, University of Gdansk, Poland, using negative staining with uranyl acetate as described previously (Gasic et al. 2011; Czajkowski et al. 2014).
Determination of bacteriophages’ host range
A host specificity assay was performed using 19 bacterial isolates of P. parmentieri collected from symptomatic potato plants and other crops in different years and countries, and on five reference/type strains of other soft rot Enterobacteriaceae viz. P. carotovorum subsp. carotovorum strain Ecc71, P. atrosepticum strain SCRI 1043, P. carotovorum subsp. brasiliense strain JJ56, Dickeya dadantii strain 3937 and D. solani strain IPO2222 as previously described (Czajkowski et al. 2014) (Table 1).
Effect of pH, temperature, chloroform, UV radiation and NaCl concentration on phage fitness under in vitro conditions
Phages were characterized for features which can be important factors in biological control applications, where phages are expected to be applied on tubers during planting, in potato rhizosphere and/or haulms to protect the growing plants against Ppa. Accordingly, they were tested for stability at a range of different pHs (pH 2 – pH 12), temperature (−20, 4, 28, 37, 42 and 85 °C) and osmolarities (different NaCl final concentrations: 0.05, 0.5, and 5.0 M NaCl) which may occur in soil environment (Czajkowski et al. 2012). Likewise, phages were tested for stability under UV light (Philips TUV G30 T8, UV dose 50 mJ cm2, 30 cm from the light source) to assess the possibility of leaf and stem applications in the field (Jones et al. 2008) and for stability in solutions containing chloroform (20% final concentration), which may be important for the isolation, purification and large scale commercial preparations of bacteriophages as reported by others (Clokie and Kropinski 2009). In each test, stability was assessed as the ratio of the bacteriophage particles which survived the experiment to the initial number of phages used. Each experiment was performed twice with the same setup and the results from both experiments were averaged. Effects of pH, temperature, chloroform, NaCl concentration and UV radiation were analyzed as previously described (Czajkowski et al. 2014).
Optimal multiplicity of infection (MOI)
Optimal multiplicity of infection was determined for phages ϕA38 and ϕA41. SCC 3193 culture in TSB (10 ml) was infected with phages at four different pfu/cfu ratios (MOI): 0.01, 0.1, 1.0 and 10.0 (1 ml). After overnight incubation at 28 °C with shaking (200 rpm), bacterial cultures were centrifuged (10,000×g, 10 min) and supernatants were assayed for phage presence as described above. The MOI resulting in the highest phage titer (the highest pfu ml−1) was considered as optimal. The experiment was independently repeated three times with the same setup and the results from all repetitions were averaged.
Effect of temperature on the optimal MOI
In order to evaluate the effect of temperature on the bacteria-phage interaction, 100 μl of 105 cfu ml−1 P. parmentieri SCC 3193 suspensions were mixed with suspensions containing individual bacteriophage (ϕA38 or ϕA41) (103 pfu ml−1) (MOI 0.01) in Ringer’s buffer (Merck). Five ml of soft top agar (TSB supplemented with 7 g l−1 agar) was added to each mixture, poured on the surface of TSA plates and incubated at four temperatures (10, 15, 22 and 28 °C) until plaques were formed. Phage titer (pfu ml−1) was calculated for each bacteriophage and each treatment. Three independent repetitions per temperature and per phage (ϕA38 or ϕA41) were performed and the results were averaged per phage. The entire experiment was performed twice with the same setup.
Phage adsorption
To determine the speed of phage adsorption to Ppa cells, 1 ml of the log-phage SCC 3193 cells (ca. 108 cfu ml−1) was infected with phage suspension to reach MOI of 0.1 (ca. 107 pfu ml−1) and incubated at 28 °C for 20 min. After 0 (control), 1, 2, 5, 10 and 20 min., two individual samples per phage were collected and centrifuged at 10000×g for 5 min to pellet the bacteria together with the adsorbed phages. The resulting supernatants were sterilized with 0.22 μm syringe filter and assayed for free, non-adsorbed phages. The experiment was repeated independently three times with the same setup and the results were averaged. Phage adsorption was calculated as previously described (Czajkowski et al. 2014).
One-step growth
To determine the latent period and burst size for ϕA38 and ϕA41, a one-step growth experiment was conducted as previously described (Ellis and Delbrück 1939; Czajkowski et al. 2014) using P. parmentieri strain SCC 3193 as a host for both bacteriophages.
Purification of bacteriophage genomic DNA for restriction fragment length polymorphism (RFLP)
Purification of phage DNA was performed using the Master Pure Complete DNA & RNA Purification Kit (Epicenter) following the manufacturer’s protocol for isolation of total DNA/RNA from cell samples. After purification, phage genomic DNA was resuspended in 20 μl of sterile demineralized water and stored at 4 °C for further use.
RFLP of phage genomes
Purified phage genomic DNA (ca. 150–400 ng μl−1) was subjected to single-enzyme restriction analysis with Csp6I, NcoI, NdeI, BamHI, HindIII, KpnI, SalI, AluI, XbaI, EcoRI, KspAI, AluI and RsaI (all from Thermo Fisher Scientific) restriction endonucleases according to the protocol provided by the manufacturer. Briefly, phage genomic DNA (ca. 200 ng per reaction) was digested for up to 24 h with 2.5 U of a restriction endonuclease in a total volume of 10 μl. Digested DNA was electrophoresed in 2% agarose gels in 0.5 x TBE. For estimation of the size of DNA fragments, λ phage genomic DNA digested with HindIII and EcoRI (Thermo Fisher Scientific) was used. Agarose gels were stained with 5 mg ml−1 of GelRed (Biotum) for visualization of digested DNA as recommended by the manufacturer.
Host challenge assay and effect of bacteriophages on potato tuber tissue maceration caused by P. parmentieri SCC3193
Host challenge assay and the assay to verify the protective effect of bacteriophages on potato tuber maceration were performed as previously described (Czajkowski et al. 2014) using P. parmentieri SCC 3193 as a host for both phages. Both experiments were repeated independently one time with the same setup and the obtained results were averaged per assay.
Statistical analysis
Data were analyzed accordingly to the experimental design used. To achieve approximate normality, counts were log-transformed adding a value 1 to avoid taking logs of zero. Effects were considered to be significant at P = 0.05 and pair-wise differences were obtained using t-test. All analyses were performed with the statistical software package Statistica v. 10 (www.statsoft.com).
Results
Isolation of P. parmentieri lytic bacteriophages from environmental samples
Between April and September 2013, 164 environmental samples were collected from different regions in Poland and assayed for the presence of lytic bacteriophages infecting P. parmentieri strain SCC 3193. After enrichment of putative bacteriophages in SCC 3193 cultures, only two samples (both from arable soil collected in Pomorskie and Lubelskie provinces, respectively, 1.22% of all samples tested) yielded lytic bacteriophages able to infect and kill exclusively P. parmentieri SCC 3193 host. From each of the two positive samples one distinct plaque was isolated and further purified to obtain pure phage particles. The obtained phages were named ϕA38 and ϕA41. Enrichment of ϕA38 and ϕA41 in SCC 3193 resulted in phage suspensions with high titer averaging 109–1010 pfu ml−1 after an overnight incubation at 28 °C. Both ϕA38 and ϕA41 formed clear plaques, ca 1.1–1.4 mm in diameter with sharp edges on lawns of P. parmentieri SCC 3193 after 24 h incubation at 28 °C.
Transmission electron microscopy (TEM) and restriction fragment length polymorphism (RFLP) analyses of ϕA38 and ϕA41
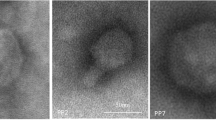
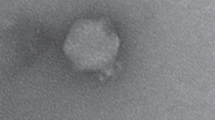
Transmission electron microscopy analysis performed for ϕA38 and ϕA41 revealed that both phages belong to the order Caudovirales and family Podoviridae based on their morphology and presence of the non-enveloped icosahedral head (diameter ca. 60 nm) (n = 10 per phage) and no-contractile short tail (length ca. 20 nm) (n = 10 per phage) (Fig. 1). Genomic DNA of phages ϕA38 and ϕA41 was insensitive for digestion with all restriction endonucleases tested, except Csp6I, for which genomic DNA obtained from phages ϕA38 and ϕA41 expressed the same restriction-nuclease patterns with all common DNA fragments in both phages (data not shown).
Transmission electron micrographs of P. parmentieri SCC 3193 bacteriophages ϕA38 and ϕA41 stained with uranyl acetate. Before microscopic analyses, the bacteriophages were purified by passaging individual plaques four times using soft top agar method and SCC 3193 as a host. ϕA38 and ϕA41 suspensions containing ca. 1016 pfu ml−1 in Ringer’s buffer (Merck) were used for staining. Each photograph represents a typical bacteriophage particle. At least 10 different photographs were taken for each sample and preparation. Scale bar: 100 nm
Host range of ϕA38 and ϕA41 phages
Both ϕA38 and ϕA41 exhibited the same host range and were able to infect 6 from 21 P. parmentieri isolates tested. The susceptible Ppa strains originated from potato samples collected in Poland, Finland and USA. Neither ϕA38 nor ϕA41 were able to infect isolates belonging to species other than P. parmentieri (Table 1).
Characterization of features involved in the stability and survival of ϕA38 or ϕA41 in the environment
Effect of pH
Stability of both phages followed the same trend. ϕA38 and ϕA41 were stable in neutral pH (pH 6.8–7.0) but their numbers were reduced both in acidic and basic pHs after incubation. A ca. 10-fold reduction of phage numbers were recorded in pH 2 and pH 12 but both ϕA38 and ϕA41 survived a 24 h incubation at each pH tested.
Effect of chloroform
Phages ϕA38 and ϕA41 were sensitive to chloroform treatment; 60 and 70% reduction of initial phage numbers were recorded for phages ϕA38 and ϕA41, respectively, after incubation in solution containing chloroform for 1 h at room temperature.
Effect of temperature
Stability of ϕA38 and ϕA41 under six different temperatures (−20, 4, 28, 37, 42 and 85 °C) followed the same trend. Both bacteriophages were more stable at lower (−20 and 4 °C) than at high temperatures (42 and 85 °C). Both phages were able to survive at 85 °C for 24 h, but incubation at this temperature resulted in at least 100-fold decrease in phage numbers.
Effect of NaCl concentrations
ϕA38 or ϕA41 were incubated for 24 h in solutions containing 0.05, 0.5 and 5.0 M NaCl or in solution without NaCl (control). No difference in phage survival between control (phages incubated without NaCl) and treatments containing NaCl was observed in both experiments.
Effect of UV radiation
ϕA38 or ϕA41 were unable to survive a 5 and 10 min exposition to UV light in repeated experiments. For both ϕA38 and ϕA41 bacteriophages, all phage particles were readily inactivated with a 5 min UV exposition.
Phage adsorption, optimal MOI and one-step growth
Adsorption of phages ϕA38 and ϕA41 to P. parmentieri strain SCC 3193 in TSB at 28 °C after first 20 min was 74 and 69%, respectively (Fig. 2a). Optimal MOI was determined to be 0.01 for both bacteriophages (data not shown). The latent period (not including the first 20 min adsorption step) was 20–25 min for both phages. The estimated average burst size for ϕA38 and ϕA41 was 102 ± 7 and 141 ± 5 ‘progeny’ phage particles per infected host cell, respectively (Fig. 2b).
Adsorption curves of bacteriophages ϕA38 and ϕA41 to P. parmentieri SCC 3193 (a) and one-step growth curves of bacteriophages ϕA38 and ϕA41 (b). a For testing the speed of phage adsorption to the bacterial host cells, 1 ml of log-phase SCC 3193 cells, 108 colony-forming units ml−1, was infected with a phage suspension to reach multiplicity of infection (MOI) 0.1 (ca. 107 plaque-forming units ml−1) and incubated at 28 °C. After incubation for 0 (control), 1, 2, 5, 10 and 20 min, two individual samples per phage were collected and centrifuged at 10000 x g for 5 min to pellet the bacteria together with the adsorbed bacteriophages. The resulting supernatants were filter sterilized with a 0.22 μm syringe filter and assayed for free, non-adsorbed phages. The experiment was repeated independently three times. b Two milliliters of exponential-growth cultures of SCC 3193 were harvested by centrifugation (10 min at 8000 x g) and resuspended in fresh tryptone soya broth (TSB) to an OD600 of 0.5 (ca. 5 × 108 colony-forming units (cfu) ml−1. A 2 ml aliquot of 5 × 108 cfu ml−1 was spiked with phage suspension (final concentration 107 plaque-forming units ml−1; multiplicity of infection, MOI = 0.1). The phages were allowed to adsorb to bacterial cells for 20 min at 28 °C. After this time, the suspension was diluted 10,000 times in TSB pre-warmed to 28 °C then incubated at 28 °C with shaking (ca. 200 rpm). Two samples of 100 μl were taken every 10 min over a period of 100 min for each phage. The number of viral particles was determined by soft top agar method. Phage plaques were counted after 24 h incubation at 28 °C
Effect of temperature on the optimal MOI
The rate of P. parmentieri SCC 3193 infection with phages ϕA38 and ϕA41 was tested at four different temperatures (10, 15, 22 and 28 °C) using bacterial cultures of 105 cfu ml−1 and viral suspensions of 103 pfu ml−1 (effective MOI 0.01). The highest ϕA38 and ϕA41 infection incidence resulting in the highest number of “progeny” bacteriophages visualized as plaques on the lawn of SCC 3193 was observed at 22 °C and 28 °C, whereas at 10 °C, seven and two-fold lower number of phage plaques in comparison with 22 °C and 28 °C was recorded for phages ϕA38 and ϕA41, respectively (data not shown).
P. parmentieri challenge in vitro assay
Initial challenge trials with P. parmentieri SCC 3193 and ϕA38 and ϕA41 bacteriophages were performed to evaluate the potential of isolated bacteriophages to act as antimicrobials against P. parmentieri. Within the first 2 h, the bacterial counts (calculated on the basis of OD600 measurement: for Ppa OD600 is equal to 108 cfu ml−1) were similar in P. parmentieri cultures infected with bacteriophages and those uninfected with ϕA38 or ϕA41 (control). After this time, the growth of bacterial cells was heavily inhibited in co-cultures containing ϕA38 or ϕA41 phages, where the number of bacteria did not increase above 107–108 cfu ml−1 during the first 12 h of incubation (Fig. 3a). In the control experiment (uninfected with bacteriophages P. parmentieri cultures), the bacterial counts reached about 109–1010 cfu ml−1 after 12 h incubation (data not shown).
Host challenge assay with P. parmentieri SCC 3193 (a) and protective effect of ϕA38 and ϕA41 bacteriophages on potato tuber tissue co-inoculated with bacteriophages and SCC 3193 (b). a ϕA38 and ϕA41 were analyzed for the ability to inhibit the growth of SCC 3193 in vitro in tryptone soya broth (TSB) at 28 °C in vitro. At time 0, 109 colony-forming units (cfu) ml−1 overnight culture of P. parmentieri SCC3193 was diluted 1:50 in fresh TSB and spiked with bacteriophages to a final concentration of ca. 105 plaque forming units ml−1. Samples were collected each hour for 12 h and used for measuring the OD600. Log (cfu + 1) ml−1 was calculated at time 12 h from the OD600 measurements and plating. For the control, a 109 cfu ml−1 overnight culture of P. parmentieri SCC 3193, diluted 1:50 in fresh TSB medium, incubated under the same conditions and processed in the same manner, was used. The experiment was repeated independently twice and the results were averaged. No bacterial colonies grew after incubation with phages for 24 h, as visualized after plating on TSA. b The effect was determined by measuring the diameter of rotting tissue (mm) after 72 h incubation at 28 °C in a humid box. Wells in potato slices were filled with: water (negative control), 106 colony-forming units (cfu) ml−1 P. parmentieri positive control, or co-inoculated with 104 plaque-forming units (pfu) ml−1 of phage ϕA38 or ϕA41 and 106 cfu ml−1 of SCC 3193. Three potato slices with three wells each, derived from three different potato tubers, were used per treatment. The experiment was repeated independently and the results were averaged. Vertical lines represent standard errors
Suppression of soft rot development in potato slices co-inoculated with SCC3193 and ϕA38 or ϕA41 bacteriophages
ϕA38 and ϕA41 were tested for their ability to reduce potato tuber tissue maceration caused by P. parmentieri SCC3193. In the replicated experiments, ϕA38 and ϕA41 bacteriophages were able to reduce potato tuber tissue maceration to at least 40–50% of that observed for the control potato slices inoculated with SCC 3193 only (Fig. 3b).
Discussion
This study was conducted to assess the presence of lytic bacteriophages infecting P. parmentieri in bulk and rhizosphere soil samples collected in different regions of Poland with the major aim to isolate new bacteriophages lytic exclusively to P. parmentieri. To the authors’ knowledge, no studies have previously documented isolation and characterization of Podoviridae bacteriophages infecting Ppa.
Of all samples analyzed, only two contained lytic bacteriophages infecting specifically Ppa. This low frequency of phage isolation was comparable to that reported in previous studies in which bacteriophages were isolated from ca. 1 to 20% of screened samples depending on the season, location, environmental conditions and presence of putative hosts (Gross et al. 1991; Marsh and Wellington 1994; Miller 2001). Moreover, also in the course of this study, enrichment of phages in host bacterial cultures was always necessary prior to isolation (Czajkowski et al. 2014, 2015).
Phages ϕA38 and ϕA41 belong to Caudovirales order and Podoviridae family assessed on their morphology as determined by the transmission electron microscopy. Furthermore, they have icosahedral heads and short tail of diameters and sizes which would classify them as C1 morphotype (Ackermann and Eisenstark 1974) of the Podoviridae family. Although different studies have shown that more than 90% of lytic bacteriophages isolated and characterized with transmission electron microscopy so far belong to the Caudovirales order (Ackermann 2003; Ackermann 1998), only ca. 14% of tailed phages belong to the Podoviridae family (Ackermann 2001). Up to date, only four SRE-infecting bacteriophage isolates viz. PP1 and PPWS1 infecting P. carotovorum subsp. carotovorum (Lee et al. 2012; Hirata et al. 2016) and Peat1 and ϕM1 infecting P. atrosepticum (Kalischuk et al. 2015; Blower et al. 2017) were classified to Podoviridae family and neither of those infects specifically P. parmentieri. This may suggest that the SRE-infecting Podoviridae bacteriophages are difficult to isolate using standard procedures and/or that the Podoviridae phages are rare in the environment (Ackermann 2011).
Phages ϕA38 and ϕA41 were indistinguishable from each other based on their morphology and RFLP profiles; they were, however, isolated from samples collected in distinct locations of Poland. There is no straightforward explanation why phenotypically and morphologically similar bacteriophages were isolated exclusively in these two agricultural regions situated ca. 500 km from each other and not in samples collected in different agricultural zones in the country. It is possible that the phages were transferred on potato tubers originating from the same location and released to soil during planting where they found alternative (soil-borne) hosts and therefore survived. However, it remains unidentified, whether the SRE-infecting bacteriophages are more frequently isolated from environments infested with the host bacteria than from the SRE-unpolluted locations (Czajkowski et al. 2015).
ϕA38 and ϕA41 were persistent under different temperatures, pH and osmolarities and when incubated in the presence of chloroform, but were immediately inactivated by the UV radiation. This suggests, as expected, that Ppa bacteriophages isolated in this study share the same pattern as other bacteriophages infecting plant pathogens (Gupta et al. 1995). Studies have demonstrated that bacteriophages are readily inactivated by exposure to sunlight and UV light, and that their populations may diminish under high temperatures, under high and low pH and under high ionic concentrations (Jones et al. 2008; Czajkowski et al. 2015).
Phages ϕA38 and ϕA41 were characterized for their latent periods, adsorption to SCC 3193 host cells and burst size. Both phages showed rapid adsorption (ca. 70% particles adsorbed in the first 20 min) and large burst size (more than 100 phages per infected cell), comparable with results obtained by others on Caudovirales bacteriophages (Weinbauer 2004). Burst size is considered as one of evolutional traits, and lytic phages which have short latency and large size of the burst may spread in bacterial population faster and hence are evolutionary more successful (Chibani-Chennoufi et al. 2004; Wang 2006; Abedon et al. 2001). The optimal multiplicity of infection (MOI) for both ϕA38 and ϕA41 phages, resulting in the highest number of ‘progeny’ phage particles, was 0.01. This optimal MOI was further influenced by temperature with the highest number of ‘progeny’ phages at relatively high temperature of 22 and 28 °C. Phage infection of host cells depends on the cell’s physiological state, which is modulated by external factors viz. temperature (Hadas et al. 1997). Whereas most in vitro research on phages is performed under optimal host growth temperature, in the natural environment the infection may take place under non-ideal conditions. According to du Raan et al. (2016) the optimal growth temperature for Ppa is 29 °C with a range from 20 to 34 °C (du Raan et al. 2016). This may suggest that ϕA38 and ϕA41 can efficiently infect Ppa in a variety of temperatures under which Ppa naturally persists in potato-associated environment.
In our study, ϕA38 and ϕA41 exhibited similar host range, being able to infect only several of Ppa isolates analyzed and no other SRE bacteria. Specifically, ϕA38 and ϕ41 were able to lyse six Ppa strains isolated from potato in Poland, Finland and USA obtained in years from 1987 to 2013 and one strain of Ppa isolated from soil in Poland in 2013, they were unable however to kill any other Ppa strains tested. Although, a number of factors determines resistance of a particular bacterium to phage infection (Hyman and Abedon 2010), there is no direct explanation why ϕA38 and ϕA41 have such a narrow ability to infect different Ppa strains. Likewise, no correlation was found between parameters such as country, plant host and year of isolation and Ppa susceptibility to ϕA38 and ϕA41 infection. Both phages were isolated initially on SCC 3193 which remains a reference strain and the most studied isolate of P. parmentieri (Nykyri et al. 2012) buy it may not necessary be the prevalent Ppa strain in potato-associated environment. Only limited data is available on the genetic diversity and ecology of Ppa (former Pwa) (Charkowski 2015; Pitman et al. 2010) and therefore more work is needed to better understand Ppa fitness under potato field conditions.
Finally, in vitro and in semi-in planta (potato slice assay) proof-of-concept experiments, ϕA38 and ϕA41 were able to inhibit the growth of Ppa and to protect potato tuber tissue from maceration caused by SCC 3193. Both phages were able to significantly reduce soft rot of potato slices in comparison with those inoculated with Ppa only. Similar protection was obtained in our previous studies when lytic bacteriophages were analyzed against D. solani (Czajkowski et al. 2014) and where broad-host bacteriophages were evaluated against combination of several SRE bacteria (Czajkowski et al. 2015) using the same potato slice assay approach. The major concern of using bacteriophages to control plant pathogens is that the phages are restricted to certain strains of target bacterium only (Jones et al. 2008, 2012). As mentioned above, ϕA38 and ϕA41 exhibit very narrow host range and therefore it is doubtful whether they could be used against Ppa in field applications alone. This could be partially overcome by using phage cocktails consisting of several phages with different host specificity against different SRE pathogens. Such approach has already been successfully introduced in veterinary (Chan et al. 2013) and food industry (García et al. 2008). In biological control of plant pathogens, examples of using phage cocktails include control of Erwinia amylovora (causing fire blight) (Gill et al. 2003), Xanthomonas campestris pv. vesicatoria (bacterial spot of tomato) (Balogh et al. 2003) and Xanthomonas axonopodis pv. citri and X. citrumelo (citrus canker, citrus bacterial spot) (Balogh et al. 2008), and Ralstonia solanacearum (bacterial wilt) (Fujiwara et al. 2011). Similarly, the number of characterized bacteriophages infecting soft rot Enterobacteriaceae increases (Czajkowski 2016) and therefore it should soon be possible to formulate a phage cocktail targeting all major soft rot and blackleg potato pathogens in one application.
In conclusion, these results indicate that ϕA38 and ϕA41 may be considered as candidates for P. parmentieri antagonists in biological control applications. Additional studies are however, required to assess the effectiveness and consistency of control in the field, phage population dynamics, proper timing of application and long-term ecotoxicological risks.
References
Abedon, S. T., (2008). Bacteriophage Ecology: Population Growth, Evolution and Impact of Bacterial Viruses. Advances in Molecular and Cellular Microbiology. Cambridge University Press.
Abedon, S. T., Herschler, T. D., & Stopar, D. (2001). Bacteriophage latent-period evolution as a response to resource availability. Applied and Environmental Microbiology, 67, 4233–4241.
Ackermann, H. W. (1998). Tailed bacteriophages: The order Caudovirales. Advances in Virus Research, 51, 135–201.
Ackermann, H. W. (2001). Frequency of morphological phage descriptions in the year 2000. Archives of Virology, 146, 843–857.
Ackermann, H. W. (2003). Bacteriophage observations and evolution. Research in Microbiology, 154, 245–251.
Ackermann, H. W. (2011). Bacteriophage taxonomy. Microbiology Australia, 91, 90–94.
Ackermann, H. W., & Eisenstark, A. (1974). The present state of phage taxonomy. Intervirology, 3, 201–209.
Adriaenssens, E. M., Van Vaerenbergh, J., Vandenheuvel, D., et al. (2012). T4-related bacteriophage LIMEstone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PloS One, 7, e33227.
Balogh, B., Jones, J. B., Momol, M. T., et al. (2003). Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Disease, 87, 949–954.
Balogh, B., Canteros, B. I., Stall, R. E., & Jones, J. B. (2008). Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Disease, 92, 1048–1052.
Bertani, G. (1951). Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. Journal of Bacteriology, 62, 293–300.
Blower, T. R., Chai, R., Przybilski, R., Chindhy, S., Fang, X., Kidman, S. E., et al. (2017). Evolution of Pectobacterium bacteriophage ΦM1 to escape two bifunctional type III toxin-antitoxin and abortive infection systems through mutations in a single viral gene. Applied and Environmental Microbiology, 83, e03229–e03216.
Chan, B. K., Abedon, S. T., & Loc-Carrillo, C. (2013). Phage cocktails and the future of phage therapy. Future Microbiology, 8, 769–783.
Charkowski, A. (2015). Biology and control of Pectobacterium in potato. American Journal of Potato Research, 92, 223–230.
Chibani-Chennoufi, S., Bruttin, A., Dillmann, M. L., & Brüssow, H. (2004). Phage-host interaction: An ecological perspective. Journal of Bacteriology, 186, 3677–3686.
Clokie, M. R. J., Kropinski, A. M., (2009). Bacteriophages: methods and protocols, volume 1: isolation, characterization, and interactions. Humana Press.
Czajkowski, R. (2016). Bacteriophages of soft rot Enterobacteriaceae – A mini-review. FEMS Microbiology Letters, 363, fnv230.
Czajkowski, R., Pérombelon, M. C. M., van Veen, J. A., & van der Wolf, J. M. (2011). Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathology, 60, 999–1013.
Czajkowski, R., de Boer, W. J., van Veen, J. A., & van der Wolf, J. M. (2012). Characterization of bacterial isolates from rotting potato tuber tissue showing antagonism to Dickeya sp. biovar 3 in vitro and in planta. Plant Pathology, 61, 169–182.
Czajkowski, R., Ozymko, Z., & Lojkowska, E. (2014). Isolation and characterization of novel soilborne lytic bacteriophages infecting Dickeya spp. biovar 3 (‘D. Solani’). Plant Pathology, 63, 758–772.
Czajkowski, R., Ozymko, Z., De Jager, V., et al. (2015). Genomic, proteomic and morphological characterization of two novel broad host lytic bacteriophages ΦPD10.3 and ΦPD23.1 infecting pectinolytic Pectobacterium spp. and Dickeya spp. PloS One, 10, e0119812.
Davidsson, P. R., Kariola, T., & Palva, E. T. (2013). Pathogenicity of and plant immunity to soft rot pectobacteria. Frontiers in Plant Science, 4, 191.
de Haan, E. G., Dekker-Nooren, T. C. E. M., van den Bovenkamp, G. W., Speksnijder, A. G. C. L., van der Zouwen, P. S., & van der Wolf, J. M. (2008). Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. European Journal of Plant Pathology, 122, 561–569.
du Raan, S., Coutinho, T. A., & nan der Waals, J. E. (2016). Cardinal temperature differences, determined in vitro, between closely related species and subspecies of pectinolytic bacteria responsible for blackleg and soft rot on potatoes. European Journal of Plant Pathology, 144, 361–369.
Duckworth, D. H., & Gulig, P. A. (2002). Bacteriophages. BioDrugs, 16, 57–62.
Ellis, E. L., Delbrück, M., (1939). The growth of bacteriophage. The Journal of General Physiology 22, 365-84.
Fujiwara, A., Fujisawa, M., Hamasaki, R., Kawasaki, T., Fujie, M., & Yamada, T. (2011). Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Applied and Environmental Microbiology, 77, 4155–4162.
García, P., Martínez, B., Obeso, J. M., & Rodríguez, A. (2008). Bacteriophages and their application in food safety. Letters in Applied Microbiology, 47, 479–485.
Gasic, K., Ivanovic, M. M., Ignjatov, M., Calic, A., & Obradovic, A. (2011). Isolation and characterization of Xanthomonas euvesicatoria bacteriophages. Journal of Plant Pathology, 93, 415–423.
Gill, J. J., Abedon, S. T., (2003). Bacteriophage ecology and plants. APSnet Feature www.apsnet.org/online/feature/phages.
Gill, J. J., Svircev, A. M., Smith, R., & Castle, A. J. (2003). Bacteriophages of Erwinia amylovora. Applied and Environmental Microbiology, 69, 2133–2138.
Goto, M., & Matsumoto, K. (1987). Erwinia carotovora subsp. wasabiae subsp. nov. isolated from diseased rhizomes and fibrous roots of Japanese horseradish (Eutrema wasabi Maxim.) International Journal of Systematic Bacteriology, 37, 130–135.
Gross, D. C., Powelson, M. L., Regner, K. M., & Radamaker, G. K. (1991). A bacteriophage-typing system for surveying the diversity and distribution of strains of Erwinia carotovora in potato fields. Phytopathology, 81, 220–226.
Gupta, K. C., Lee, Y. A., & Yin, J. (1995). Extremo-phage: In vitro selection of tolerance to a hostile environment. Journal of Molecular Evolution, 41(2), 113–114.
Hadas, H., Einav, M., Fishov, I., & Zaritsky, A. (1997). Bacteriophage T4 development depends on the physiology of its host Escherichia coli. Microbiology, 143, 179–185.
Hinton, J. C. D., Sidebotham, J. M., Hyman, L. J., Pérombelon, M. C. M., & Salmond, G. P. C. (1989). Isolation and characterisation of transposon-induced mutants of Erwinia carotovora subsp. atroseptica exhibiting reduced virulence. Molecular and General Genetics MGG, 217, 141–148.
Hirata, H., Kashihara, M., Horiike, T., Suzuki, T., Dohra, H., Netsu, O., et al. (2016). Genome sequence of Pectobacterium carotovorum phage PPWS1, isolated from Japanese horseradish [Eutrema japonicum (Miq.) Koidz] showing soft-rot symptoms. Genome Announcements, 4, e01625–e01615.
Hugouvieux-Cotte-Pattat, N., & Robert-Baudouy, J. (1994). Molecular analysis of the Erwinia chrysanthemi region containing the kdgA and zwf genes. Molecular Microbiology, 11, 67–75.
Hyman, P., & Abedon, S. T. (2010). Bacteriophage host range and bacterial resistance. Advances in Applied Microbiology, 70, 217–248 Academic Press, Elsevier.
Jones, J. B., Jackson, L. E., Balogh, B., Obradovic, A., Iriarte, F. B., & Momol, M. T. (2008). Bacteriophages for plant disease control. Annual Review of Phytopathology, 45, 245–262.
Jones, J. B., Vallad, G. E., Iriarte, F. B., et al. (2012). Considerations for using bacteriophages for plant disease control. Bacteriophage, 2, 208–214.
Kalischuk, M., Hachey, J., & Kawchuk, L. M. (2015). Complete genome sequence of phytopathogenic Pectobacterium atrosepticum bacteriophage Peat1. Genome Announcements, 13, e00760–e00715.
Khayi, S., Cigna, J., Chong, T. M., Quêtu-Laurent, A., Chan, K.-G., Hélias, V., & Faure, D. (2016). Transfer of the potato plant isolates of Pectobacterium wasabiae to Pectobacterium parmentieri sp. nov. International Journal of Systematic and Evolutionary Microbiology, 66, 5379–5383.
Lapwood, D. H., & Harris, R. I. (1982). The spread of Erwinia carotovora subsp. atroseptica and subsp. carotovora from stem lesions and degenerating seed tubers to progeny tubers in soil. Potato Research, 25, 41–50.
Lapwood, D. H., & Read, P. J. (1986). The susceptibility of potato stems of different potato cultivars to blackleg caused by Erwinia carotovora subsp. atroseptica. Annals of Applied Biology, 109, 555–560.
Lee, J. H., Shin, H., Ji, S., et al. (2012). Complete genome sequence of phytopathogenic Pectobacterium carotovorum subsp. carotovorum bacteriophage PP1. Journal of Virology, 86, 8899–8900.
Marsh, P., & Wellington, E. M. H. (1994). Phage-host interactions in soil. FEMS Microbiology Ecology, 15, 99–107.
Miller, R. (2001). Environmental bacteriophage-host interactions: Factors contribution to natural transduction. Antonie Van Leeuwenhoek, 79, 141–147.
Nykyri, J., Niemi, O., Koskinen, P., et al. (2012). Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium parmentieri SCC3193. PLoS Pathogens, 8, e1003013.
Pérombelon, M. C. M. (2002). Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathology, 51, 1–12.
Pirhonen, M., Heino, P., Helander, I., Harju, P., & Palva, E. T. (1988). Bacteriophage T4 resistant mutants of the plant pathogen Erwinia carotovora. Microbial Pathogenesis, 4, 359–367.
Pitman, A. R., Harrow, S. A., & Visnovsky, S. B. (2010). Genetic characterisation of Pectobacterium wasabiae causing soft rot disease of potato in New Zealand. European Journal of Plant Pathology, 126, 423–435.
Pritchard, L., Glover, R. H., Humphris, S., Elphinstone, J. G., & Toth, I. K. (2016). Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Analytical Methods, 8, 12–24.
Toth, I. K., van der Wolf, J. M., Saddler, G., et al. (2011). Dickeya species: An emerging problem for potato production in Europe. Plant Pathology, 60, 385–399.
Twest, R., Kropinski, A. M., (2009). Bacteriophage enrichment from water and soil. In: Clokie MJ, Kropinski A, eds. Bacteriophages. Humana Press, 15–21. (Methods in Molecular Biology™; vol. 501).
van der Wolf, J. M., & de Boer, S. H. (2007). Bacterial pathogens of potato. Potato Biology and Biotechnology, Advances and Perspectives (pp. 595–619). Oxford: Elsevier.
van der Wolf, J. M., de Haan, E. G., Kastelein, P., Krijger, M., de Haas, B. H., Velvis, H., et al. (2017). Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathology, 66(4), 571–583. doi:10.1111/ppa.12600.
Waleron, M., Waleron, K., & Lojkowska, E. (2013). Occurrence of Pectobacterium parmentieri in potato field samples. European Journal of Plant Pathology, 137, 149–158.
Wang, I. (2006). Lysis timing and bacteriophage fitness. Genetics, 172, 17–26.
Weinbauer, M. G. (2004). Ecology of prokaryotic viruses. FEMS Microbiology Reviews, 28, 127–181.
Wiken Dees, M., Lebecka, R., Perminow, J. I. S., Czajkowski, R., Grupa, A., Motyka, A., Zoledowska, S., Sliwka, J., Lojkowska, E., & Brurberg, M. B. (2017). Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. European Journal of Plant Pathology. doi:10.1007/s10658-016-1140-2).
Willis, J. W., Engwall, J. K., & Chatterjee, A. K. (1987). Cloning of genes for Erwinia carotovora subsp. carotovora pectolytic enzymes and further characterization of the polygalacturonases. Phytopathology, 77, 1199–1205.
Yuan, K., Li, X., Adam, Z., et al., (2014). Comparative genomics for reclassification of P. wasabiae subspecies. In. The 3rd International Erwinia Workshop. Shanghai, page 27.
Acknowledgements
The work was financially supported by the National Center for Research and Development (Narodowe Centrum Badan i Rozwoju - NCBR), Poland via a LIDER program research grant (LIDER/450/L-6/14/NCBR/2015) to Robert Czajkowski. The authors would like to thank Sylwia Jafra and Magdalena Rajewska (University of Gdansk, Poland) for helpful discussions and Jacquie van der Waals (University of Pretoria, South Africa) for providing the P. carotovorum subsp. brasiliense strain JJ56 for the bacteriophages’ host specificity assay.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are fully aware of this submission and have declared that no competing interests exist.
Human and animal participants
This study did not involve any human and/or animal participants.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Smolarska, A., Rabalski, L., Narajczyk, M. et al. Isolation and phenotypic and morphological characterization of the first Podoviridae lytic bacteriophages ϕA38 and ϕA41 infecting Pectobacterium parmentieri (former Pectobacterium wasabiae). Eur J Plant Pathol 150, 413–425 (2018). https://doi.org/10.1007/s10658-017-1289-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1289-3