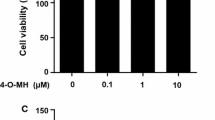
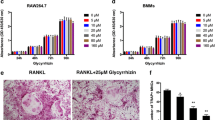
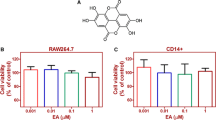
Abstract
Osteoclasts are multinucleated cells that play a crucial role in bone resorption, and are formed by the fusion of mononuclear osteoclasts derived from osteoclast precursors of the macrophage lineage. Compounds that specifically target functional osteoclasts would be ideal candidates for anti-resorptive agents for clinical applications. In the present study, we investigated the effects of luteolin, a flavonoid, on the regulation of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis, functions and signaling pathway. Addition of luteolin to a coculture system of mouse bone marrow cells and ST2 cells in the presence of 10−8 M 1α,25(OH)2D3 caused significant inhibition of osteoclastogenesis. Luteolin had no effects on the 1α,25(OH)2D3-induced expressions of RANKL, osteoprotegerin and macrophage colony-stimulating factor mRNAs. Next, we examined the direct effects of luteolin on osteoclast precursors using bone marrow macrophages and RAW264.7 cells. Luteolin completely inhibited RANKL-induced osteoclast formation. Moreover, luteolin inhibited the bone resorption by mature osteoclasts accompanied by the disruption of their actin rings, and these effects were reversely induced by the disruption of the actin rings in mature osteoclasts. Finally, we found that luteolin inhibited RANKL-induced osteoclastogenesis through the suppression of ATF2, downstream of p38 MAPK and nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1 (NFATc1) expression, respectively. Taken together, the present results indicate that naturally occurring luteolin has inhibitory activities toward both osteoclast differentiation and functions through inhibition of RANKL-induced signaling pathway as well as actin ring disruption, respectively.





Similar content being viewed by others
References
Asagiri M, Sato K, Usami T et al (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269
Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Phamacol Ther 90:157–177
Boyle WJ, Simonet WS, Laccey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Chambers TJ (2000) Regulation of the differentiation and function of osteoclasts. J Pathol 192:4–13
Chambers TJ, Magnus CJ (1982) Calcitonin alters behavior of isolated osteoclasts. J Pathol 136:27–39
Cobb MH, Goldsmith EJ (1995) How MAP kinase are regulated. J Biol Chem 270:14843–14846
Comalada Mònica, Ballester Isabel, Bailòn Elvira et al (2006) Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem Pharmacol 72:1010–1021
Darnay BG, Aggarwal BB (1999) Signal transduction by tumor necrosis factor and tumor necrosis factor related ligands and their receptors. Ann Rheum Dis 58:I2–I13
Darnay BG, Haridas V, Ni J et al (1998) Characterization of the intracellular domain of receptor activator of NF-κB (RANK): interaction with tumor necrosis factor receptor-associated factors and activation of NF-κB and c-Jun N-terminal kinase. J Biol Chem 273:20551–20555
De Smet PA (2002) Herbal remedies. N Engl J Med 347:2046–2056
Gohil K, Packer L (2002) Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann N Y Acad Sci 957:70–77
Hotokezaka H, Sakai E, Kanaoka K et al (2002) U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem 277:47366–47372
Ikeda F, Nishimura R, Matsubara T et al (2004) Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest 114:475–484
Kim JS, Jobin C (2005) The flavonoid luteolin prevents lipopolysaccharide-induced NF-κB signaling and gene expression by blocking IκB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology 115:375–387
Kim SH, Shin KJ, Kim D et al (2003) Luteolin inhibits the nuclear factor-κB transcriptional activity in Rat-1 fibroblasts. Biochem Pharmacol 66:955–963
Kim K, Kim JH, Lee J et al (2005) Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem 208:35209–35216
Koga T, Inuli M, Inoue K (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428:758–763
Kotanidou A, Xagorari A, Bagli E (2002) Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am J Respir Crit Care Med 165:818–823
Lakkakorpi PT, Väänänen HK (2004) Calcitonin, prostaglandin E2, and dibuturyl cyclic adenosine 3′, 5′-monophosphate disperse the specific microfilament structure in resorbing osteoclasts. J Histochem Cytochem 38:1487–1493
Lerner UH (2004) New molecules in the tumor necrosis factor ligand and receptor super families with importance for physiological and pathological bone resorption. Crit Rev Oral Biol Med 15:64–81
Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M (2000) Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). J Biol Chem 275:31155–31161
Matsumoto M, Kogawa M, Wada S (2004) Essential role of p38 mitogene-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem 279:45969–45979
Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Mocasi A, Humphrey MB, van Ziffle JA et al (2004) The immunomodulatory adapter proteins DAP12 and Fc receptor gamma chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA 101:6158–6163
Nakamura I, Takahashi N, Sasaki T et al (1991) Wortmannin, a specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett 361:79–84
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Ross JA, Kasum CM (2002) Dietary flavonoids, bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34
Sharma SM, Bronisz A, Hu R et al (2007) MITF and PU.1 recruit p38 MAPK and NFATc1 to target genes during osteoclast differentiation. J Biol Chem 282:15921–15929
Suda T, Takahashi N, Martin TJ (1991) Modulation of osteoclast differentiation. Endocr Rev 13:66–80
Suda T, Jimi E, Nakamura I et al (1997) Role of 1α, 25-dihydroxyvitaminD3 in osteoclast differentiation and function. Methods Enzymol 282:223–235
Suzuki H, Nakamura I, Takahashi N et al (1996) Calcitonin-induced changes in the cytoskeleton are mediated by a signal pathway associated with protein kinase A in osteoclasts. Endocrinology 137:4685–4690
Takayanagi H (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Takayanagi H (2005) Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83:170–179
Takayanagi H (2007) Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304
Teitlebaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Teitelbaum SL (2004) RANKing c-jun in osteoclast development. J Clin Invest 114:463–465
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Väänänen HK, Zhao H, Mulari M et al (2000) The cell biology of osteoclast function. J Cell Sci 113:377–381
Walsh MC, Kim N, Kadono Y et al (2006) Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24:33–63
Whitmarsh AJ, Davis RJ (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathway. J Mol Med 74:589–607
Wong BR, Josein R, Lee SY et al (1998) The TRAF family of sigal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem 273:28355–28359
Xagorari A, Papapetropoulos A, Mauromatis A et al (2001) Luteolin inhibits an endotoxi-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophage. J Pharmacol Exp Ther 296:181–187
Ziyan L, Yongmei Z, Nan Z et al (2007) Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med 73:221–226
Acknowledgments
This study was supported by an endowment from Erina Co. Inc. and partially funded by a grant for Biodefense Programs of the Ministry of Education, Science and Technology of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JW., Ahn, JY., Hasegawa, Si. et al. Inhibitory effect of luteolin on osteoclast differentiation and function. Cytotechnology 61, 125–134 (2009). https://doi.org/10.1007/s10616-010-9253-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-010-9253-5