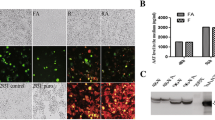
Abstract
Objectives
Alpha-1 antitrypsin (A1AT) deficiency is associated with emphysema and liver disease. Only plasma-derived A1AT protein is available for augmentation therapy. Recombinant A1AT (recA1AT) protein expressed in various types of available hosts are either non-glycosylated or aberrantly glycosylated resulting into reduced stability and biological activity. To overcome these limitations, we have used the human liver HepG2 cell line to produce recA1AT protein.
Results
HepG2 cells were transfected by A1AT cDNA and cell populations were generated that stably overexpressed A1AT protein. Real-time RT-PCR and rocket immunoelectrophoresis of cell culture supernatants indicated that the transfection resulted more than two-fold increase in A1AT production compared to that of control parental cells. Immunoblot analysis showed that both plasma and HepG2-produced A1AT proteins have identical molecular weight in either glycosylated or deglycosylated form. Partial digestion with PNGase F indicated that the three N-glycosylation sites of recA1AT, like the native A1AT protein in plasma, are occupied. Recombinant A1AT also like the native A1AT was thermostable and could efficiently inhibit trypsin proteolytic activity against BSA and BAPNA chromogenic substrate. The recombinant HepG2 cells cultured in media containing B27 serum free supplement released recA1AT at the same level as in the serum containing media.
Conclusions
RecA1AT production in HepG2 cells grown under serum free condition at a large scale could provide a reliable source of the native protein suitable for therapeutic use in human.






Similar content being viewed by others
References
Agarwal S, Singh R, Sanyal I, Amla DV (2008) Expression of modified gene encoding functional human alpha-1-antitrypsin protein in transgenic tomato plants. Transgenic Res 17:881–896
Bischoff R, Speck D, Lepage P, Delatre L, Ledoux C, Brown SW, Roitsch C (1991) Purification and biochemical characterization of recombinant alpha 1-antitrypsin variants expressed in Escherichia coli. Biochemistry 30:3464–3472
Blanchard V, Liu X, Eigel S, Kaup M, Rieck S, Janciauskiene S, Sandig V, Marx U, Walden P, Tauber R, Berger M (2011) N-glycosylation and biological activity of recombinant human alpha1-antitrypsin expressed in a novel human neuronal cell line. Biotechnol Bioeng 108:2118–2128
Campos-da-Paz M, Costa CS, Quilici LS, de Carmo Simões I, Kyaw CM, Maranhão AQ, Brigido MM (2008) Production of recombinant human factor VIII in different cell lines and the effect of human XBP1 co-expression. Mol Biotechnol 39:155–158
Castilho A, Windwarder M, Gattinger P, Mach L, Strasser R, Altmann F, Steinkellner H (2014) Proteolytic and N-glycan processing of human α1-antitrypsin expressed in Nicotiana benthamiana. Plant Physiol 166:1839–1851
Dietz AA, Rubinstein HM, Hodges LK (1976) Use of alpha-N-benzoyl-l-arginine-p-nitroanilide as trypsin substrate in estimation of alpha 1-antitrypsin. Clin Chem 22:1754–1755
Ekeowa UI, Gooptu B, Belorgey D, Hägglöf P, Karlsson-Li S, Miranda E, Pérez J, MacLeod I, Kroger H, Marciniak SJ, Crowther DC, Lomas DA (2009) Alpha1-antitrypsin deficiency, chronic obstructive pulmonary disease and the serpinopathies. Clin Sci (Lond) 116:837–850
Garver RI Jr, Chytil A, Courtney M, Crystal RG (1987) Clonal gene therapy: transplanted mouse fibroblast clones express human alpha 1-antitrypsin gene in vivo. Science 237:762–764
Ghouse R, Chu A, Wang Y, Perlmutter DH (2014) Mysteries of α1-antitrypsin deficiency: emerging therapeutic strategies for a challenging disease. Dis Model Mech 7:411–419
Gordon EM, Tang H, Salazar RL, Kohn DB (1993) Expression of coagulation factor IX (Christmas factor) in human hepatoma (HepG2) cell cultures after retroviral vector-mediated transfer. Am J Pediatr Hematol Oncol 15:196–203
Guzdek A, Potempa J, Dubin A, Travis J (1990) Comparative properties of human alpha-1-proteinase inhibitor glycosylation variants. FEBS Lett 272:125–127
Hang GD, Chen CJ, Lin CY, Chen HC, Chen H (2003) Improvement of glycosylation in insect cells with mammalian glycosyltransferases. J Biotechnol 102:61–71
Kalsheker N (1989) Alpha 1-antitrypsin: structure, function and molecular biology of the gene. Biosci Rep 9:129–138
Karnaukhova E, Ophir Y, Golding B (2006) Recombinant human alpha-1 proteinase inhibitor: towards therapeutic use. Amino Acids 30:317–332
Kataoka H, Seguchi K, Inoue T, Koono M (1993) Properties of alpha 1-antitrypsin secreted by human adenocarcinoma cell lines. FEBS Lett 328:291–295
Kwon KS, Song M, Yu MH (1995) Purification and characterization of alpha 1-antitrypsin secreted by recombinant yeast Saccharomyces diastaticus. J Biotechnol 42:191–195
Laurell CB (1966) Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem 15:45–52
Lee KJ, Lee SM, Gil JY, Kwon O, Kim JY, Park SJ, Chung HS, Oh DB (2013) N-glycan analysis of human α1-antitrypsin produced in Chinese hamster ovary cells. Glycoconj J 30:537–547
McCarthy C, Saldova R, Wormald MR, Rudd PM, McElvaney NG, Reeves EP (2014) The role and importance of glycosylation of acute phase proteins with focus on alpha-1antitrypsin in acute and chronic inflammatory conditions. J Proteom Res 13:3131–3143
Naghibalhossaini F, Pakdel A, Ghaderi AA, Saberi Firoozi M (2005) Effective production of carcinoembryonic antigen by conversion of the membrane-bound into a recombinant secretory protein by site-specific mutagenesis. Pathol Oncol Res 11:211–217
Pakdel A, Naghibalhossaini F, Mokarram P, Jaberipour M, Hosseini A (2012) Regulation of carcinoembryonic antigen release from colorectal cancer cells. Mol Biol Rep 39:3695–3704
Ross D, Brown T, Harper R, Pamarthi M, Nixon J, Bromirski J, Li CM, Ghali R, Xie H, Medvedeff G, Li H, Scuderi P, Arora V, Hunt J, Barnett T (2012) Production and characterization of a novel human recombinant alpha-1-antitrypsin in PER.C6 cells. J Biotechnol 162:262–273
Schuetz EG, Schuetz JD, Strom SC, Thompson MT, Fisher RA, Molowa DT, Li D, Guzelian PS (1993) Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology 18:1254–1262
Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ (2003) Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307:573–582
Wang Z, Hilder TL, van der Drift K, Sloan J, Wee K (2013) Structural characterization of recombinant alpha-1-antitrypsin expressed in a human cell line. Anal Biochem 437:20–28
Acknowledgments
This study was a part of the dissertation of Hajar Jaberie, submitted to Shiraz University of Medical Sciences in partial fulfillment of the requirements for the Ph. D in clinical biochemistry. This work was supported by a grant from the Vice Chancellor for Research, Shiraz University of Medical Sciences (Grant Number 92-6699).
Supporting information
Supplementary Table 1—Primers’ sequence used for the related RT-PCR reactions.
Supplementary Fig. 1—The 1257 base pair full length A1AT RT-PCR product (panel A, lane 1) was cloned into pcDNA3.1 expression vector. The presence of the insert was confirmed by digestion of pcDNA3.1-A1AT construct with HindIII/EcoRI restriction enzymes (panel B, lane 2). M: DNA size marker
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2016_2150_MOESM2_ESM.tif
Supplementary material 2 (TIFF 202 kb) Supplementary Fig. 1. The1257 base pair full length A1AT RT-PCR product (panel A, lane 1) cloned into pcDNA3.1 expression vector. The presence of the insert was confirmed by digestion of pcDNA3.1-A1AT construct with HindIII/EcoRI restriction enzymes (panel B, lane 2). M: DNA size marker
Rights and permissions
About this article
Cite this article
Jaberie, H., Naghibalhossaini, F. Recombinant production of native human α-1-antitrypsin protein in the liver HepG2 cells. Biotechnol Lett 38, 1683–1690 (2016). https://doi.org/10.1007/s10529-016-2150-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2150-z