Abstract
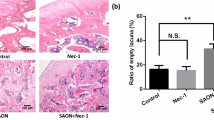
Steroid-induced avascular necrosis of the femoral head (SANFH) is a major limitation of long-term or excessive clinical administration of glucocorticoids. Fludarabine, which is a compound used to treat various hematological malignancies, such as chronic lymphocytic leukemia, acts by down-regulating signal transducer and activator of transcription 1 (STAT1) by inhibiting STAT1 phosphorylation in both normal and cancer cells. This study assessed the effects of fludarabine in vitro (primary murine osteoblasts) and in vivo (rat SANFH model). In vitro, pretreatment with fludarabine significantly inhibited Dexamethasone (Dex)-induced apoptosis in osteoblasts, which was examined by TUNEL staining. Treatment with Dex caused a remarkable decrease in the expression of Bcl-2; an increase in cytochrome c release; activation of BAX, caspase-9, and caspase-3; and an obvious enhancement in STAT1 phosphorylation. However, treatment resulted in the up-regulation of caspase-3 expression. Enhanced P-STAT1 activity and up-regulation of caspase-3 expression were also observed in osteoblasts. In vivo, the subchondral trabeculae in fludarabine-treated rats exhibited less bone loss and a lower ratio of empty lacunae. Taken together, our results suggest that STAT1-mediated up-regulation of caspase-3 is involved in osteoblast apoptosis induced by Dex and indicates that fludarabine may serve as a potential agent for the treatment of SANFH.









Similar content being viewed by others
References
Schäcke H, Döcke WD, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96(1):23–43
Den UD, Bultink IE, Lems WF (2011) Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep 13(3):233–240
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365(1):62–70
Kerachian MA, Séguin C, Harvey EJ (2009) Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol 114(3–5):121–128
Murata M et al (2007) Osteonecrosis in stroke-prone spontaneously hypertensive rats: effect of glucocorticoid. J Orthop Sci 12(3):289–295
Souttou B, Raulais D, Vigny M (2001) Pleiotrophin induces angiogenesis: Involvement of the phosphoinositide-3 kinase but not the nitric oxide synthase pathways. J Cell Physiol 187(1):59–64
Himburg HA et al (2010) Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med 16(4):475–482
Fan JB et al (2015) EGFR-AKT-mTOR activation mediates epiregulin-induced pleiotropic functions in cultured osteoblasts. Mol Cell Biochem 398(1):105–113
Yun SI et al (2009) Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3β. J Bone Miner Metab 27(2):140–148
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17(17):2481–2495
Kim S et al (2007) Essential role for signal transducer and activator of transcription-1 in pancreatic beta-cell death and autoimmune type 1 diabetes of nonobese diabetic mice. Diabetes 56(10):2561–2568
Allagnat F et al (2011) Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to β-cell apoptosis. Cell Death Differentiation 18(2):328–337
Tajima K et al (2010) Inhibition of STAT1 accelerates bone fracture healing. J Orthop Res 28(7):937–941
Zhang J et al (2000) Spontaneous thymocyte apoptosis is regulated by a mitochondrion-mediated signaling pathway. J Immunol 165(6):2970–2974
Kumar A et al (1997) Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278(5343):1630–1632
Lu K, Wang X (2012) Therapeutic advancement of chronic lymphocytic leukemia. J Hematol Oncol 5(1):1–12
Chaudhuri A, Persidsky Y (2007) STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood–brain barrier. In International Symposium on Neurovirology
Hui Z et al (2009) Radiosensitization by inhibiting STAT1 in renal cell carcinoma. Int J Radiat Oncol Biol Phys 73(1):288–295
Kaur T et al (2010) Short interfering RNA against STAT1 attenuates cisplatin-induced toxicity in the rat by suppressing inflammation. Cell Death Dis 2(7):e180
Mukherjea D et al (2011) NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss. Antioxid Redox Signal 14(6):999
Dong Y et al (2015) Systemic application of teriparatide for steroid induced osteonecrosis in a rat model. BMC Musculoskelet Disord 16(1):1–8
Yamamoto T et al (1997) Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheumatol 40(11):2055–2064
Qin L et al (2008) Multiple bioimaging modalities in evaluation of an experimental osteonecrosis model induced by a combination of lipopolysaccharide and methylprednisolone. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chin J Repar Reconstr Surg 22(3):258–264
Low SC et al (2014) External and internal bone micro-architecture in normal and Kienbock’s lunates: a whole-bone micro-computed tomography study. J Orthop Res 32(6):826–833
Torella D et al (2007) Fludarabine prevents smooth muscle proliferation in vitro and neointimal hyperplasia in vivo through specific inhibition of STAT-1 activation. Am J Physiol Heart Circ Physiol 292(292):H2935–H2943
Chaudhuri A et al (2008) STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood–brain barrier. Blood 111(4):2062–2072
Jr JJ (1993) Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Related Res 292(292):294–308
Wang TY, Avlonitis EG, Relkin R (1988) Systemic necrotizing vasculitis causing bone necrosis. Am J Med 84(6):1085–1086
Xu X et al (2014) STAT1–caspase 3 pathway in the apoptotic process associated with steroid-induced necrosis of the femoral head. J Mol Hist 45(4):473–485
Calder JDF et al (2004) Apoptosis—a significant cause of bone cell death in osteonecrosis of the femoral head. Bone Joint J 86(8):1209–1213
Chua CC et al (2003) Dexamethasone induces caspase activation in murine osteoblastic MC3T3-E1 cells. Biochim Et Biophysica Acta 1642(1–2):79–85
Nomura M et al (1999) Apoptotic cytosol facilitates Bax translocation to mitochondria that involves cytosolic factor regulated by Bcl-2. Cancer Res 59(21):5542–5548
Hu WP et al (2007) DC-81-Indole conjugate agent induces mitochondria mediated apoptosis in human melanoma A375 cells. Chem Res Toxicol 20(6):905–912
Borutaite V, Brown GC (2003) Mitochondria in apoptosis of ischemic heart. FEBS Lett 541(1–3):1–5
Ramana CV et al (2000) Complex roles of Stat1 in regulating gene expression. Oncogene 19(21):2619–2627
Kim TH et al (2007) Peroxisome proliferator-activated receptor-gamma gene polymorphisms are not associated with osteonecrosis of the femoral head in the Korean population. Molecules Cells 24(3):388–393
Acknowledgements
The authors thank the staff of the Laboratory of the Orthopaedic Research Institute and the Scientific Research Center of the Second Affiliated Hospital of Wenzhou Medical University. This work was generously supported by grants from the Zhejiang Province Public Welfare Technology Application Research Project, China (2015C33209) and Wenzhou Public Welfare Science and Technology Project, Zhejiang Province, China (Y20150243).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Feng, Z., Zheng, W., Tang, Q. et al. Fludarabine inhibits STAT1-mediated up-regulation of caspase-3 expression in dexamethasone-induced osteoblasts apoptosis and slows the progression of steroid-induced avascular necrosis of the femoral head in rats. Apoptosis 22, 1001–1012 (2017). https://doi.org/10.1007/s10495-017-1383-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1383-1